RESEARCH TOPICS
Transient chirality inversion during racemization
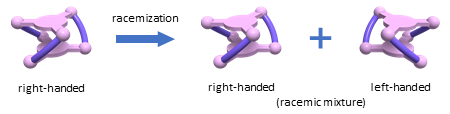
Many biologically-important molecules, such as amino acids, have right- and left-handed forms (chirality) that are not superimposable with their mirror images. The two forms may have different biological activities. They can be separated from each other, but when separated, some of them may undergo racemization, i.e., equilibration to give a mixture of the right- and left-handed forms in a 50:50 ratio (Fig 1). During the racemization process, the ratio of the right- and left-handed forms decreases monotonically from 100% to 50%. For example, during the racemization of an isolated right-handed form, the left-handed form cannot exceed the right-handed form.
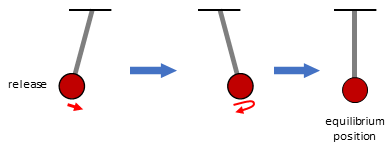
On the other hand, in our daily life, there are many examples of “pendulum”-like motions, in which an object moves to the opposite side beyond the equilibrium position then finally stops at the equilibrium position (Fig 2). If the racemization of a chiral molecule proceeds with a time-dependent change similar to the pendulum-like motion, the right-handed form is transiently converted into the left-handed form then becomes the racemic form.
A helical molecular cage, metallocryptand, which contains three cobalt(III) ions, was found to show interesting time-dependent change like the above-mentioned pendulum motion (Fig 3). This molecule can be functionalized with six amine molecules, which are exchangeable with different kinds of amine molecules. In this study, we introduced six molecules of chiral amine A to bias the chirality toward right-handed. Since this chiral amine A molecules are introduced, the right- and left-handed forms of the metallocryptand become a diastereomer pair, which have different stabilities.
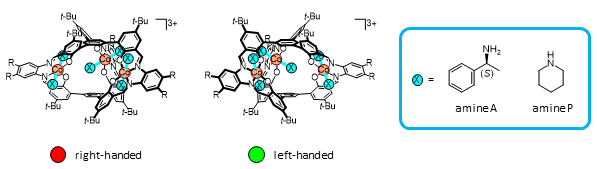
When another amine P without chirality was added to this metallocryptand to remove the chiral amine A, the right-handed forms gradually changed to be racemic. During the process, the left-handed form became dominant before the complete racemization (Fig 4,5). Detailed spectroscopic analysis of the reaction indicated that the right-to-left inversion occurred after replacement of four molecules of chiral amine A with achiral amine P and that the two “A” ligands at each cobalt(III) center are replaced at virtually the same time.
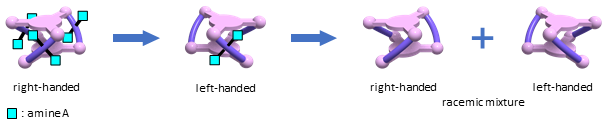
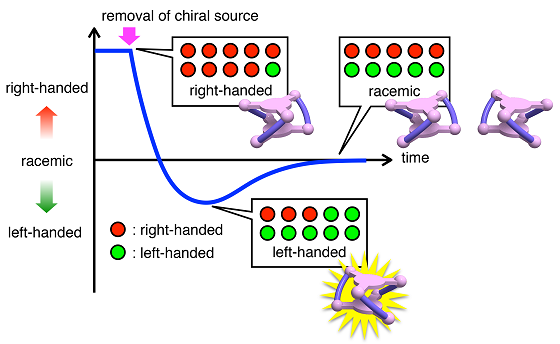
Interestingly, such unique right/left inversion was not observed in the reverse process, i.e., the exchange of achiral amine P in the racemic metallocryptand by chiral amine A. During the exchange reaction, the right-handed form monotonically increased without inversion, thus the right-handed form was always dominating. Detailed spectroscopic analysis indicated that the amine exchange (P→A) in the metallocryptand proceeded in a statistically random manner. Consequently, the left-handed form appears only in the forward step. Obviously, the forward (A→P) and reverse (P→A) exchange processes constitute a hysteretic cycle.
In general, the time course of most chemical reactions obeys the first-order reaction kinetics. The A→P conversion in this study showed a unique time course change with chirality inversion followed by racemization. The sign inversion before reaching the final equilibrium state is seen in the “damped oscillation” in physical phenomena such as the pendulum motion and the “overshoot” in control theory. In chemical reactions, however, such a unique time change has been observed only when multiple molecules interact in a complicated manner, such as the autocatalytic reaction of inorganic ions such as Belousov-Zhabotinsky's oscillating reaction and iodine clock reaction, and the formation and/or conversion process of supramolecular polymers. In this research, we first observed a unique time response in which the right-handed form is transiently switched to the left-handed form before the complete racemization on a simple single-molecular platform, without relying on complicated multi-molecular assemblies.
[Reference]
“Transient chirality inversion during racemization of a helical cobalt(III) complex”
Sakata, Y.; Chiba, S.; Akine, S.
Proc. Natl. Acad. Sci. USA 2022, 119, e2113237119
doi:10.1073/pnas.2113237119

