RESEARCH TOPICS
Oligometal template strategy:
Selective synthesis of macrocycles with a defined size
Macrocyclic multinucleating ligands, which have multiple coordination sites in a macrocyclic structure, are useful to obtain multi-metal structures containing multiple metal ions in a predictable arrangement. In general, however, the synthesis of macrocyclic organic compounds is not efficient, mainly because the final step of the intramolecular macrocyclization often compete with intermolecular reaction such as polymerization. In the supramolecular chemistry, template synthesis strategy has been successfully utilized to synthesize macrocyclic host molecules. In the template-directed syntheses, the final step of the macrocyclization becomes more efficient in the presence of a suitable guest metal ion. We adopted this strategy to synthesize macrocyclic multinucleating ligands by using multiple metal ions as the template.
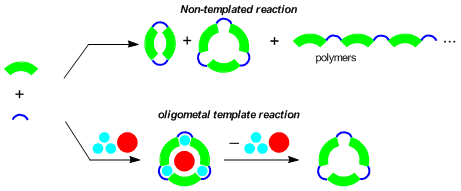
We have investigated the synthesis and complexation behavior of a series of macrocyclic ligands having three salen-type coordinating moieties. We already reported that tris(saloph) macrocycles (R = o-C6H4) can be obtained in high yield (91%) without using template metal ions. However, the corresponding tris(salamo) ligand (R = OCH2CH2O) was obtained in very low yield (less than 15%) in the absence of template ions.
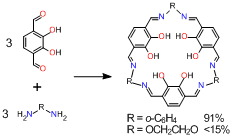
Instead, the reaction gave acyclic and cyclic oligomers with different sizes.
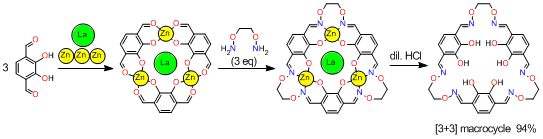
The yield of the [3+3] macrocycle was surprisingly improved (94%) when we used
template ions,
Zn2+ and La3+, which have high affinity toward this type of macrocyclic ligands.
The significantly strong template effect can be attributable to the suitable arrangement of
organic constituent molecules fixed with the aid of the metal ions.
The oligometallic template strategy is also applicable to the synthesis of macrocycles
in which the final cyclization step is C=C bond formation as well as C=N bond formation.
Indeed, we have synthesized several kinds of macrocyclic oligonucleating ligands containing C=C bonds
by Ru-catalyzed olefin metathesis on the basis of the oligometallic template strategy.
[References]
“Core/Shell Oligometallic Template Synthesis of Macrocyclic Hexaoxime”
Akine, S.; Sunaga, S.; Taniguchi, T.; Miyazaki, H.; Nabeshima, T.
Inorg. Chem. 2007, 46, 2959-2961.
doi:10.1021/ic062327s
“Oligometallic Template Strategy for Ring-Closing Olefin Metathesis:
Highly Cis- and Trans-Selective Synthesis of a 32-Membered Macrocyclic Tetraoxime”
Akine, S.; Kagiyama, S.; Nabeshima, T.
Inorg. Chem. 2007, 46, 9525-9527.
doi:10.1021/ic701585x

