RESEARCH TOPICS
A capped molecular container for control of uptake/release of guest ions
Macrocyclic host molecules with a cavity can be regarded as a nano-sized container, because we can put various kinds of guest molecules and ions in their cavity. Guest uptake and release process by most of simple host molecules is known to be very fast on the human timescale (within milliseconds), i.e., guests are freely entering and exiting the cavity. Therefore, we can say that these host molecules behave as containers without caps. On the other hand, macroscopic containers used in our daily life may have caps, by which we can open and close the containers (Fig 1). However, it has been difficult to incorporate such a trivial function into molecular-sized containers.
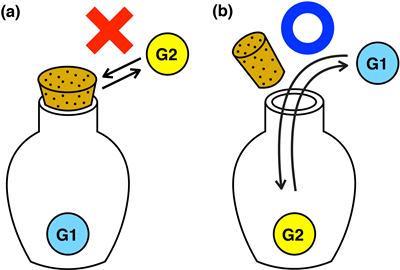
It is important to develop a strategy to open/close molecular containers in researches on molecular containers from the viewpoint of their potential application to storing, transport, and release of functional molecules. It is anticipated to develop a mechanism for suppressing and accelerating guest uptake/release on the human timescale (seconds - minutes - hours).
In this research, we designed a new macrocyclic host that has caps at the portals (Fig 2). By using this molecule, we developed a new host-guest system in which we can control the kinetics of ion uptake/release triggered by exchange of the caps.
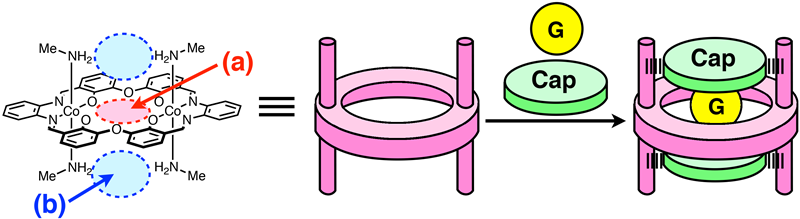
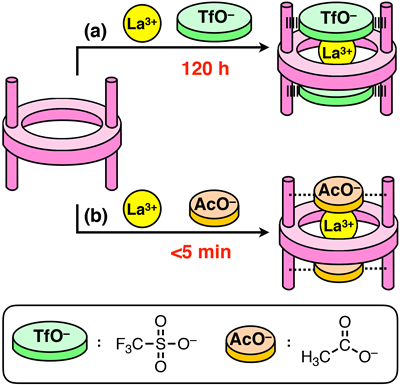
We synthesized a metallohost molecule, which has four methylamine (CH3NH2) ligands and a cation recognition site based on crown-ether-like scaffold. This host molecule recognized various kinds of metal ions (Na+, K+, Rb+, Cs+, Ca2+, La3+) in the crown-ether-like cavity. At the same time, the counter anions (triflate anion, CF3SO3–) were introduced at the capping sites (see Fig 2. (b)). These triflate anions are hydrogen-bonded to the methylamine molecules of the metallohost, which was evidenced by the X-ray crystallographic analysis (Figs 3,4).
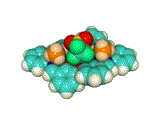
[Hover to animate]
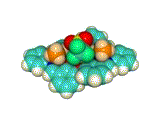
[Hover to animate]
As describe above, the guest uptake of usual macrocyclic host molecules such as crown ethers is very fast. In contrast, the guest uptake of the metallohost in this study was very slow. In particular, the complexation with La(OTf)3 was extremely slow; it took 120 h for complete encapsulation. The retarded guest inclusion should mainly be attributed to the effect of anion caps. This uptake rates can be tuned by changing the counteranions (Fig 5). When acetate ion (CH3CO2–) is used as a cap, the uptake of La3+ was completed within 5 min. The uptake was at least 100 times faster than that for TfO–.
Since the counteranions significantly affect the guest uptake/release rates, we expected that exchanges of the anion caps would trigger the guest uptake/release. The triflate anion caps are weakly bound via hydrogen bonds, it would be easy to replace the triflate ion with other anions. Based on this idea, we tried to develop a system in which we can exchange guest cations in an on-demand manner.
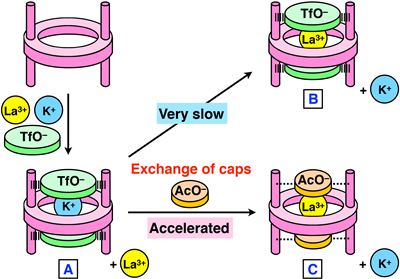
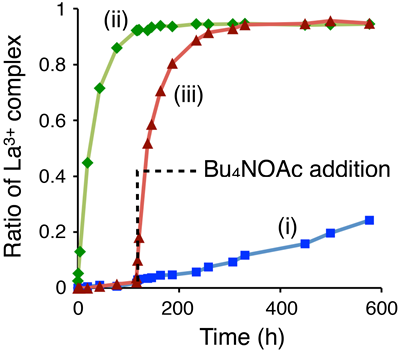
We chose K+ and La3+, which are taken up quickly and slowly, respectively. When both ions were added to the metallohost in the presence of triflate caps, only K+ was taken up in the cavity of the metallohost (Fig 6, A). Our binding (thermodynamic) studies had shown that La3+ forms more stable host-guest complex than K+, but the conversion of the K+-complex to the more stable La3+ complex did not occur (Fig 6, B). This indicates that the triflate caps introduced in the metallohost almost completely suppress the exchange of the guests in the cavity. Interestingly, the guest ion exchange proceeded much more quickly when acetate ion is used instead of the triflate ion; the reaction was accelerated by ~75 times (Fig 6, C), which was estimated from the initial rates. It is noteworthy that we can accelerate this metal exchange at any stage of the reaction. When AcO– was added after 120 h, the guest exchange started and the La3+ complex immediately increased (Fig 7). Therefore, the acetate ion acted as a trigger to initiate the guest exchange from the kinetically trapped state.
To date, many kinds of stimuli-responsive guest uptake/release or guest exchange systems have been developed. In most cases, their switching mechanism relies on the reversal of the thermodynamic stabilities in the two different states (Fig 8). The stimuli-responsive guest exchange of our host molecule is based on the difference in the guest uptake kinetics without changing the binding strengths (Fig 9). The anion caps enabled us to make a very stable kinetically trapped state, from which we can initiate the guest ion exchange at any stage of the reaction using anion cap exchange as a trigger. It is well-known that non-equilibrium phenomena play an important role in biological systems, as seen in the control of ion concentrations by passive transport. Such a complicated ion upkate/release control was achieved by using only one simple molecule, the cobalt metallohost in our study.

[Hover to animate]

[Hover to animate]
[Reference]
“Anion-capped metallohost allows extremely slow guest uptake and on-demand acceleration of guest exchange”
Sakata, Y.; Murata, C.; Akine, S.
Nat. Commun. 2017, 8, 16005.
doi:10.1038/ncomms16005

