RESEARCH TOPICS
Inverting helical structures:
-- Helix inversion by molecular leverage mechanism
Helical structure can adopt either a right-handed or left-handed form. If we can convert one of them into the other in response to external stimuli, the helical structures will work as a switching system that can change their chiral functions. To date, there have been reported several examples of helicity inversion systems based on helical organic framework, metal complexes, polymers, etc., in which the helicity inversion takes place in response to external stimuli. However, it is generally difficult to change the helicity as we like in a predictable fashion. Our group aims to develop new strategies to invert the helicity of helical structures using totally predictable mechanisms based on finely designed organic frameworks.

[Hover to animate]
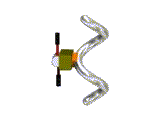
We have conceived of a new idea to invert the helical structure by utilizing a
totally predictable leverage-like mechanism.
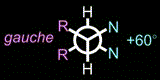
This mechanism is based on a gauche/anti conversion of a tetrasubstituted ethane derivative.
We introduced two substituents as handles of a leverage into the -CH2CH2- moiety
of one of the three salen-type coordination moiety of the helical complex (R groups in the Figure).
If we shorten the distance between the two R groups, the N-C-C-N dihedral angle becomes +60 deg,
and if we extend the distance, the angle becomes -60 deg.
This means that changing the R-R distance should cause the inversion of the dihedral angle,
which would change the helicity of the entire molecule.
[Hover to animate]
[Hover to animate]
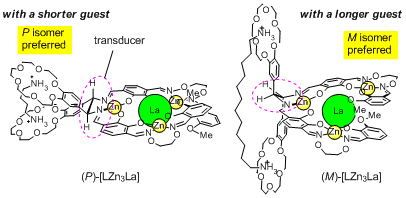
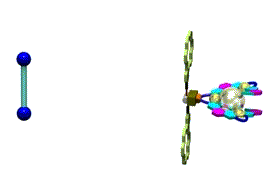
We chose a crown ether derivative as a handle of the leverage, in order to change the helicity by using molecular recognition of ammonium derivatives. When we added a shorter diammonium cation, the right-handed form became dominant, and longer diammonium cation caused a helix inversion to give the left-handed helix as we expected. This complex is the first helix inversion system based on a molecular machinery mechanism, and the first example that can change the information of "molecular length" into the information of "helicity".
[Hover to animate]
[Hover to animate]
[Reference]
“A Molecular Leverage for Helicity Control and Helix Inversion”
Akine, S.; Hotate, S.; Nabeshima, T.
J. Am. Chem. Soc. 2011, 133, 13868-13871.
doi:10.1021/ja205570z

