RESEARCH TOPICS
A molecular cage that can close the aperture
As we use “containers” in our daily life, there are “molecular containers”, e.g., “molecular cages”, in nano-world. The cavity of the molecular cages is well separated from the outside environment, and the guest uptake and release are generally slow due to the three-dimensional scaffold. In fact, whether or not the guest species are entrapped permanently depends on the size of the cage portals compared to the guest size. Nanosized molecular containers that can close their aperture depending on specific situations would be advantageous when we use these containers for storing and transporting functional molecules (Fig 1).

[Hover to animate]
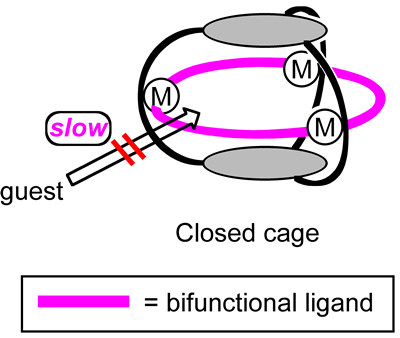
We focused on the reversible feature of metal−ligand coordination bonds, which allow us to introduce bifunctional ligands at the apertures of molecular cages to control entering of the guest species (Fig 2). Since the bifunctional ligands have to be introduced without destroying the cage skeleton, we should use a robust cage framework that can remain intact under the conditions for coordination bond formation/cleavage (Fig 3).

[Hover to animate]
In this context, tris(saloph) organic cage H6L is advantageous to this study when compared to coordination cages. If we introduce hexacoordinate metal ions into its saloph tetradentate sites, we can introduce two additional ligands at the axial positions of each metal. This allows us to introduce a bifunctional ligand at each aperture of the molecular cage. The bifunctional ligands can bridge neighboring metal centers to close the aperture, which should kinetically suppress the guest uptake (Fig 4).
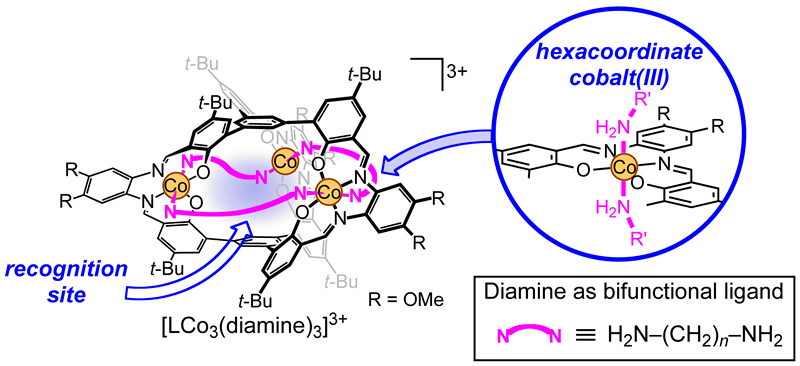
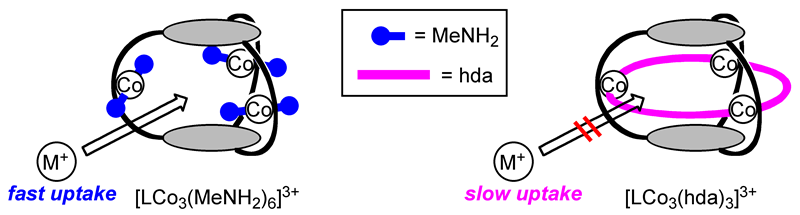
In this study, we used the low-spin d6 cobalt(III) ion, which generally forms hexacoordinate inert complexes and undergoes very slow ligand exchange. This would enable facile introduction of the diamine ligand that is firmly fixed at the apertures of the molecular cage without affecting the organic cage skeleton. The closed-cage trinuclear complex with three 1,6-hexanediamine (hda) ligands, [LCo3(hda)3](OTf)3, was synthesized in high yield. We also synthesized the open-cage analogue, [LCo3(MeNH2)6](OTf)3. The aperture of this open-cage complex [LCo3(MeNH2)6]3+ can be easily closed by a reaction with the bifunctional ligand hda. X-ray crystallographic analysis of [LCo3(hda)3]3+ revealed that the three hda ligands, which bridge the three cobalt ions, are effectively blocking the apertures to give a closed-cage (Fig 5). Nevertheless, there should remain enough space for guest inclusion inside the cage of [LCo3(hda)3]3+ (Fig 6). The three cobalt(III) ions and three hda ligands constitute a 27-membered (Co-NH2-(CH2)6-NH2)3 macrocycle. The cavity is surrounded by two pivotal benzene rings in parallel as well as six phenoxo oxygen atoms forming a triangular prism.

[Hover to animate]
[Hover to animate]
Prior to evaluating the effect of the bifunctional ligands on the guest-uptake kinetics, we investigated the ion recognition behavior of the open-cage complex [LCo3(MeNH2)6](OTf)3 toward alkali metal ions (Na+, K+, Rb+, Cs+). The host showed Cs+ selectivity (Cs+ > Rb+ > K+ >> Na+) and the guest inclusion quickly reached equilibrium (within 5 min). The rate constants for guest binding (k+, k–) were roughly estimated as follows: k+ > 4 M–1s–1, k– > 2 x 10–4 s–1. We then investigated the blocking effect of the diamine ligands in the closed-cage complex [LCo3(hda)3](OTf)3 on its binding kinetics. In this case, [LCo3(hda)3](OTf)3 showed no uptake of Cs+ after 1 h. It took approximately 50 h for the 40% conversion to the Cs+ complex. The uptake rate for the closed-cage complex (k+ ≈ 2 x 10–3 M–1s–1) was lower by at least 2000 times than that for the open-cage complex (k+ > 4 M–1s–1). The guest release of the closed-cage complex (k– ≈ 5 x 10–6 s–1) was also significantly retarded compared to that of the open-cage complex (k– ≈ 2 x 10–4 s–1). The hda ligands in [LCo3(hda)3]3+ effectively block the guest uptake by more than 3 orders of magnitude (Fig 7). The concept of the open−close feature in this system would be applied to a molecular container in which the entrapped molecules can be released when required.
[Reference]
“A Metallo-molecular Cage That Can Close the Apertures with Coordination Bonds”
Akine, S.; Miyashita, M.; Nabeshima, T.
J. Am. Chem. Soc. 2017, 139, 4631–4634.
doi:10.1021/jacs.7b00840

