RESEARCH TOPICS
Making helical structures by spontaneous processes
Helical structures are inherently chiral; they have a right- or left-handed helicity. If we can obtain helical structures in a simple one-step procedure from a linear organic molecule, the conversion would be useful to regulate molecular functions that are associated with the helical structures. We have been investigating such a spontaneous helix formation process by taking advantage of the labile character of metal ions. We designed a series of linear chain-like molecules that can form helical structures upon metal complexation.
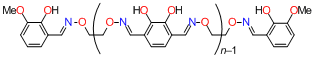
The molecules have a structure in which several salen-type coordination sites are linked together. When d-block metal ions are added, formation of four coordination bonds at each salen-type coordination site makes the entire molecule adopt a helical structure. Since the resultant helical metal complexes have a lot of phenoxo oxygen atoms directing inside, they are expected to strongly bind to additional metal ions.

[Hover to animate]
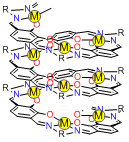
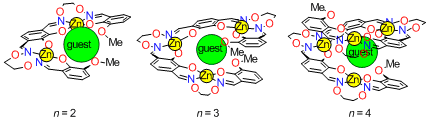
We found that the salen-oligomer ligands (n = 2,3,4) formed a helical structure upon complexation with zinc(II) acetate when a hard metal ion (Ca2+, lanthanide, etc.) is present. The hard metal ions are strongly bound in the helical cavity surrounded by oxygen atoms. The helical complexes existed as an equilibrated mixture of right- and left-handed forms, and the complex having longer oligomer ligands underwent slower helix inversion. The helix inversion rates also depended on the central metal ions. For example, the [LZn3La]3+ underwent much slower helix inversion than [LZn3Ba]2+. Such a dynamic feature of the helical metal complexes would be useful to design a system in which the structural conversion can dynamically change various kinds of chiral functions.
[References]
“Novel Synthetic Approach to Trinuclear 3d-4f Complexes: Specific Exchange of the
Central Metal of a Trinuclear Zinc(II) Complex of a Tetraoxime Ligand with a Lanthanide(III) Ion”
Akine, S.; Taniguchi, T.; Nabeshima, T.
Angew. Chem. Int. Ed. 2002, 41, 4670-4673.
doi:10.1002/anie.200290011
“Ca2+- and Ba2+-Selective Receptors Based on Site-Selective
Transmetalation of Multinuclear Polyoxime-Zinc(II) Complexes”
Akine, S.; Taniguchi, T.; Saiki, T.; Nabeshima, T.
J. Am. Chem. Soc. 2005, 127, 540-541.
doi:10.1021/ja046790k
“Guest-dependent Inversion Rate of a Tetranuclear Single Metallohelicate”
Akine, S.; Taniguchi, T.; Matsumoto, T.; Nabeshima, T.
Chem. Commun. 2006, 4961-4963.
doi:10.1039/b610641b
“Helical Metallohost-Guest Complexes via Site-Selective Transmetalation of Homotrinuclear Complexes”
Akine, S.; Taniguchi, T.; Nabeshima, T.
J. Am. Chem. Soc. 2006, 128, 15765-15774.
doi:10.1021/ja0646702
“Synthesis of Acyclic Tetrakis- and Pentakis(N2O2) Ligands
for Single-helical Heterometallic Complexes with a Greater Number of Winding Turns”
Akine, S.; Matsumoto, T.; Sairenji, S.; Nabeshima, T.
Supramol. Chem. 2011, 23, 106-112.
doi:10.1080/10610278.2010.514906

