RESEARCH TOPICS
Metal complexes that can overcome statistical complexity
Heterometallic complexes are of particular interest among metal-containing substances when designing multifunctional catalysts or magnetic materials. During the synthesis of heterometallic complexes, however, we sometimes encounter difficulty that we rarely experience in organic syntheses. In organic syntheses, we can make molecules basically at will by using chemoselective and regioselective reactions. Once covalent bonds are formed, they do not undergo bond recombination. On the other hand, coordination compounds often undergo bond recombination, which leads to statistical complexity.
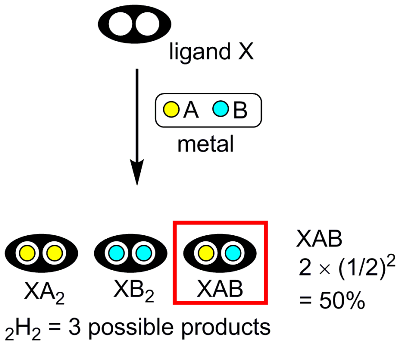
As the simplest case, we consider a ligand X with two binding sites (Fig 1), each of which can accommodate two different kinds of metals, A or B. If a homodinuclear species XA2 is needed, it can be obtained simply by reacting X with 2 equiv of A. However, if a heterodinuclear species XAB is needed, it is not always obtained as the sole product in the reaction of X with a mixture of A and B. The reaction could give three (2H2 = 3)[note 1] products, XA2, XB2, and XAB. If there is no preference in reactivity between A and B, the yield of the XAB species is governed by statistics: 2 x (1/2)2 = 50%. Nevertheless, we might isolate the desired XAB species from the mixture because separation of only three products is not particularly difficult in many cases.
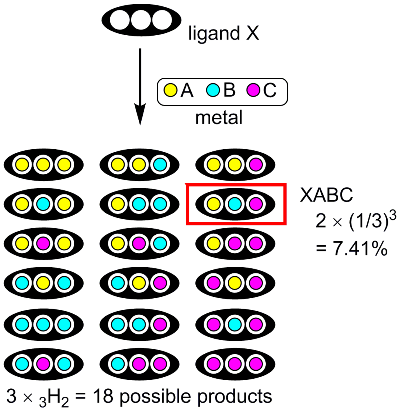
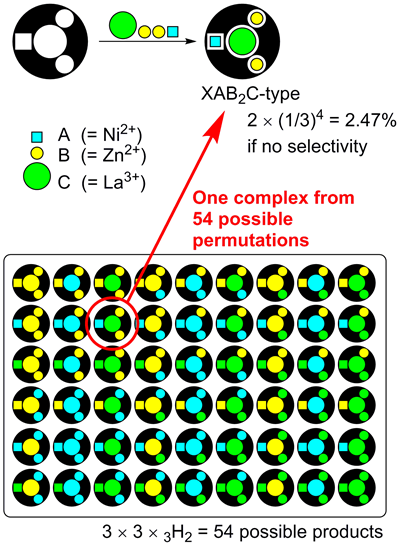
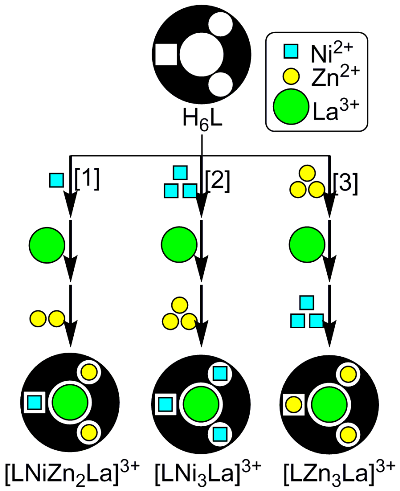
As the number of binding sites increases and a greater variation of metals are used, the one-step synthesis of multi-metal structures becomes more and more difficult. We here consider a three-site ligand X, which can accommodate three different metals, A, B, and C with equal probability (Fig 2). The reaction could give 18 (3 x 3H2 = 18) products. As the expected yield of XABC species is less than 10% (2 x (1/3)3 = 7.41%), most of the substrates (>90%) will be lost as undesired side products. Another factor that makes it difficult to obtain multi-metal structures is the reversibility of the coordination bonds between metals and organic ligands. For example, a heterospecies XABC may be in equilibrium with other competing 17 species such as XABA, XBCA, etc. For this reason, to date, there are very limited examples of metal complexes containing three different metals. Obviously, we need a strategy to overcome statistical complexity in order to introduce different kinds of metal ions in a programmed fashion. A four-site ligand H6L (Fig 3) would be suitable for the selective metal introduction. It has four metal-binding sites (one salen, two salamo, and one O8 sites). Surprisingly, the complexation of this ligand H6L with Ni2+, Zn2+, and La3+ ions selectively gave only one [LNiZn2La]3+ among the 54 possible complexes as the thermodynamically most stable product.
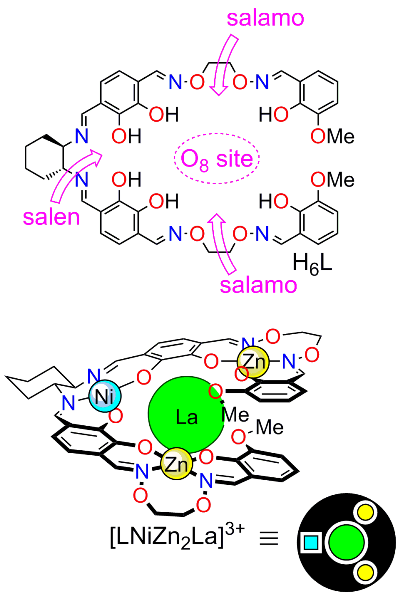
The complex [LNiZn2La]3+ was obtained in high yield by the introduction of 1 equiv of Ni2+ followed by the reaction with 2 equiv of Zn2+ and 1 equiv of La3+ (Fig 6 [1]). The structure of [LNiZn2La]3+ was determined by X-ray crystallographic analysis (Fig 5) and spectroscopic techniques.
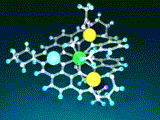
[Hover to animate]
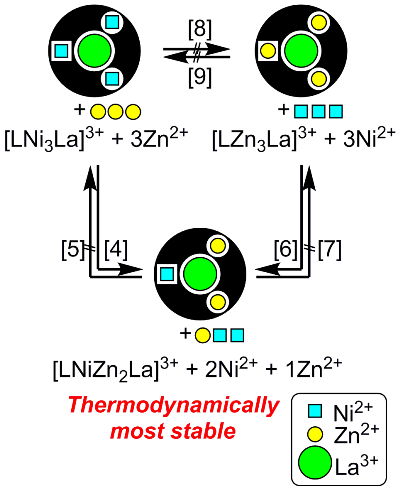
However, different kinds of tetranuclear complexes ([LZn3La]3+ and [LNi3La]3+) were formed upon the the addition of Zn2+ and Ni2+ in different orders (Fig 6 [1], [2], [3]). This result suggests that metal ions are not freely exchangeable under the synthetic conditions of these complexes. We could know whether the trimetallic species [LNiZn2La]3+ is the most thermodynamically stable product if the complexes are heated in the presence of sufficient amounts of Zn2+ and Ni2+. Indeed, [LNiZn2La]3+ was always the favored product when [LNi3La]3+ was heated with 3 equiv of Zn2+ (Fig 7 [4]) or [LZn3La]3+ was heated with 3 equiv of Ni2+ (Fig 7 [6]). On the other hand, [LNiZn2La]3+ remained intact when it was heated with 2 equiv of Ni2+ and 1 equiv of Zn2+ (Fig 7 [5],[7]). Therefore, [LNiZn2La]3+ was formed even if metals were initially introduced in different sites.
Thus, we have developed the first molecular system that can selectively accommodate
three kinds of metal ions in specified positions.
The XAB2C-type complex [LNiZn2La]3+ was solely and spontaneously formed
among 54 (= 3 × 3 × 3H2) possible products upon heating.
The different characteristics of the three kinds of coordination sites in H6L
allowed the selective introduction of three different metals and thereby
allowed us to overcome the statistical complexity.
To date, studies on multimetallic systems containing three or more kinds of metal ions
have been limited to inert metal systems mainly owing to the lack of versatile synthetic strategies,
despite the interest in such complexes because of the interplay that occurs between the different metals.
Multicoordination strategies based on finely designed ligands such as H6L
could pave the way for the development of a wide range of multimetallic functional systems
with different kinds of metal ions.
[Note 1] The nHr notation denotes the number of combinations of n distinct objects
taking r at a time when objects may occur with repetitions.
This can be calculated as
(n + r - 1)!/((n - 1)!r!).
[Reference]
"Overcoming Statistical Complexity: Selective Coordination of Three Different Metal Ions
to a Ligand with Three Different Coordination Sites"
Akine, S.; Matsumoto, T.; Nabeshima, T.
Angew. Chem. Int. Ed. 2016, 55, 960-964
(Hot Paper; highlighted as an Inside Back Cover).
doi:10.1002/anie.201508065

