RESEARCH TOPICS
Switching of ion recognition mechanisms in reactive host-guest systems
Molecules that can capture specific guests (e.g., molecules or ions) are referred to as hosts. The binding with the guest by the host is known as molecular or ion recognition because the host recognizes the shape and size of the guest.
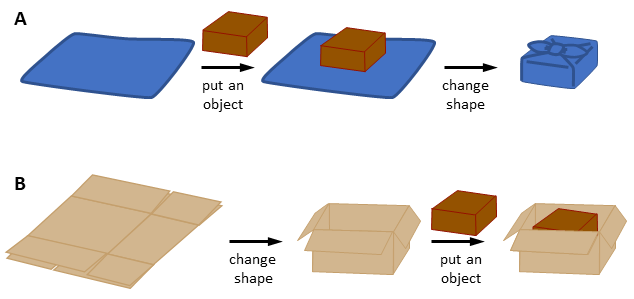
Actually, the host molecule changes its shape to fit the guest during the host-guest binding processes, just like a sheet of wrapping paper for an object. This is known as “induced fit”, which is frequently observed in the enzyme-substrate binding in biochemical studies (Fig 1A). Recently, another pathway “conformational selection” has also attracted increasing attention, in which the conformational change of an enzyme occurs before the substrate binding (Fig 1B). Guest binding of artificial host molecules may occur in these two different pathways, but it seemed difficult to differentiate these two mechanisms because both the guest binding and structural change are completed instantly. Therefore, most of the conformational changes in artificial host molecules upon guest binding are regarded as “induced fit” without taking into account the possibile alternative pathway of the “conformational selection”. The question of whether the conformational change occurs before or after the guest binding does not seem to be important. In our daily life, however, there are two types of “wrapping” methods: changing the shape of a wrapping paper after putting an object (Fig 1A) and preparing a foldable box before putting an object (Fig 1B). The difference in the order sometimes makes a big difference in the results; for example, a mousetrap will not properly work if you close it before a mouse enters.
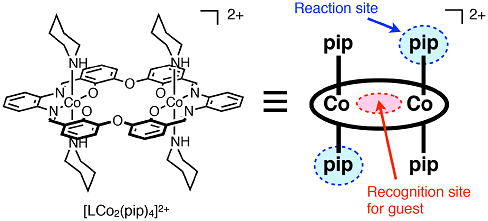
In this study, we first successfully switched the two different recognition pathways of guest binding associated with a chemical reaction. We designed a new macrocyclic cobalt(III) metallohost molecule, which can undergo chemical reaction and guest recognition. The molecule contains four piperidine ligands, which allow ligand exchange reaction with different kinds of ligands. In addition, the macrocyclic molecule has a cavity surrounded by six oxygen atoms, which is suitable for the recognition of alkali metal ions as observed for crown ethers (Fig 2).
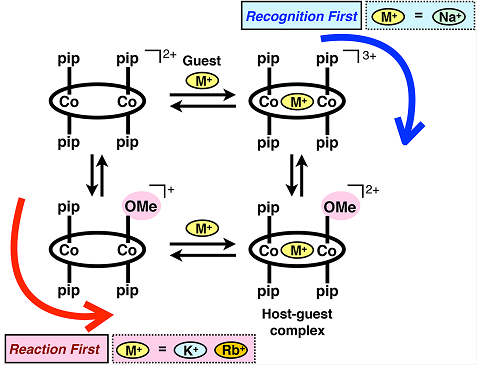
When Na+ was added to this cobalt(III) metallohost, Na+ was bound to the metallohost and, at the same time, two of the four piperidine ligands were replaced with MeO ligands. This means that both “reaction” and “guest binding” took place upon the Na+ addition. In contrast to the quick enzymatic conformational changes, the ligand exchange reaction of the metallohost is slow with the timescale of minutes to hours. This allows us to spectroscopically analyze the reaction in detail, and concluded that the Na+ binding occurred by the recognition-first mechanism, i.e., the Na+ binding occurs before the ligand exchange reaction, because the ligand exchange was significantly accelerated upon increase of the Na+ concentration. On the other hand, the ligand exchange was not significantly accelerated when K+ and Rb+ was added, and this indicated that the binding with K+ and Rb+ occurred by the different mechanism, reaction-first mechanism, i.e., the ligand exchange with MeO occurs at first and then the guest is bound. Therefore, the recognition pathways can be switched by changing the guest cations (Fig 3). The combination of the guest binding and chemical reactions would be useful to design responsive functional molecules and time-programmable systems.
[Reference]
“Switching of Recognition First and Reaction First Mechanisms in Host–Guest Binding Associated with Chemical Reactions”
Sakata, Y.; Tamiya, M.; Okada, M.; Akine, S.
J. Am. Chem. Soc. 2019, 141, 15597-15604.
doi:10.1021/jacs.9b06926

