Prologue
Inorganic chemistry is a fun
If you want to be a pioneer in a field, if you want to explore your own science, chemistry might be the one you want to pursue. In inorganic chemistry, there is a lot of stuff left undiscovered, and you find the land of opportunity to catch your curiosity. When we learn science, digging it really deep, you may be overwhelmed by the impossibility. To achieve your goal, a big budget more than one university can afford and an elite team of professional PhDs may be the must. Unlike a big science, chemistry is a treasure island waiting your participation as an independent investigator. If you are going to explore unknown territory, unprecedented world, you have a chance to establish your own chemistry at Kanazawa University, by yourself.
The land of unclaimed peaks
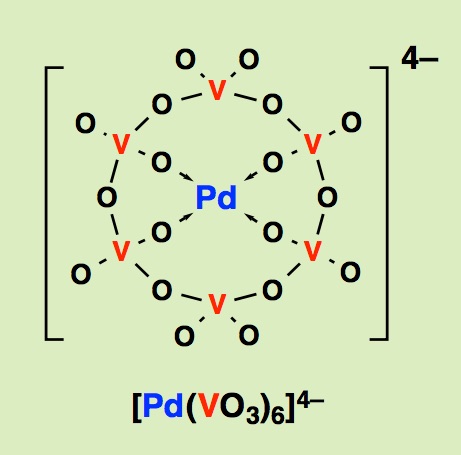
Ask any one who knows chemistry, "Do you know a molecule that has more than six carbon atoms?" It is an easy question even for a high school student who learns chemistry. Benzene, toluene, fullerenes, you name it. We can immediately think of a dozen of examples, showing the maturity of organic chemistry. All right, then, how about any molecule that has more than six particular metals? It is a tough question even for a graduate student in chemistry department. If you have an answer, your talents are impressive and welcome to join us. Only a specialist may know such a complex. This situation should be altered. It is not impossible to create a molecule, such as Cr6, Fe6 complexes and so on, even if it is a random guess. These imaginary metal cluster complexes should be an important precursor for a future high-tech material. Here, the problem is, not how important such a complex is, rather we simply do not know how to create it. We explore such a possibility through the study of polyoxometalate chemistry, that is a molecular entity of metal oxides.