• Condensation Reaction of Oxoacids
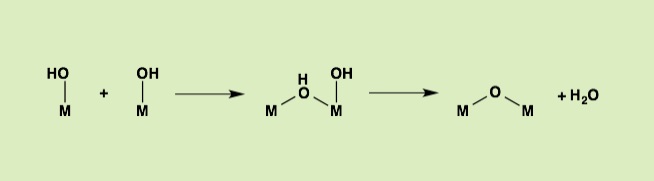
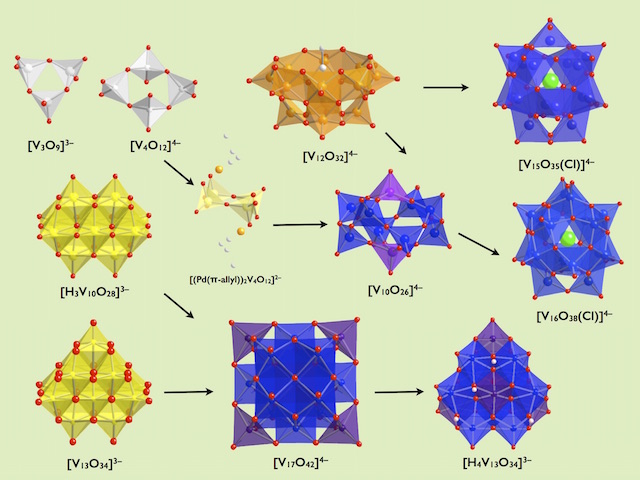
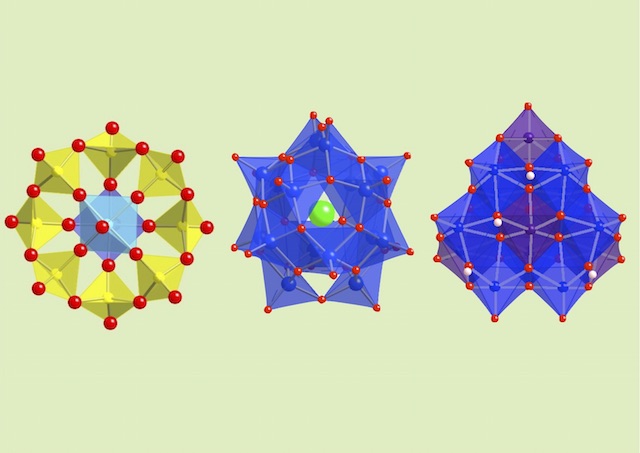
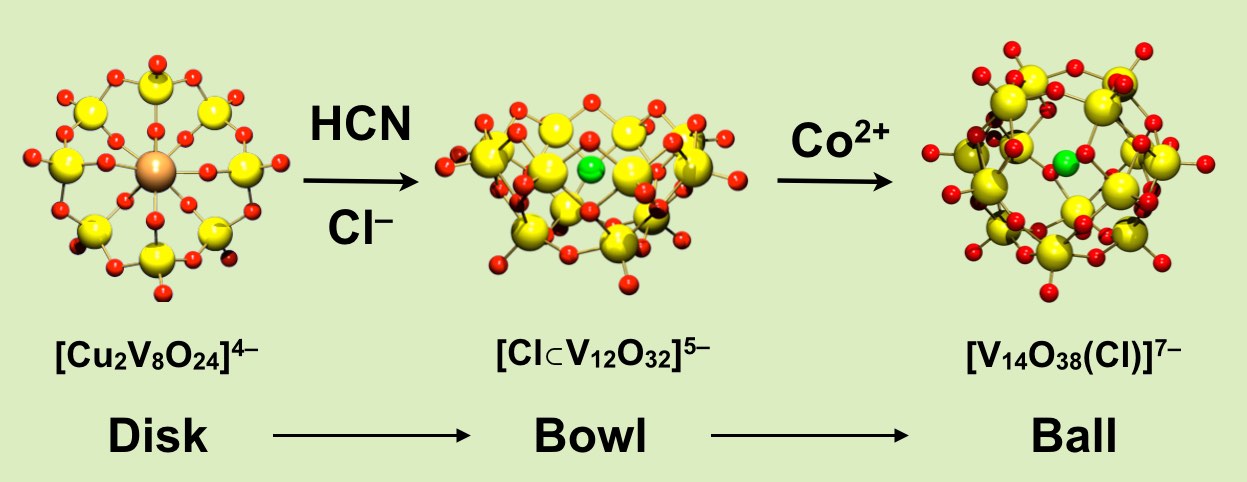
The oxoacids of molybdenum, tungsten and vanadium grow from a mononuclear species into a nano-molecule by acid condensation and water elimination reactions, and it results in the formation of polyoxometallates. The precise stoichiometric adjustments with a controlled amount of water and acids, drive the reaction to proceed to make a solution species favourable for a particular anionic framework. The first step is a protonation on a terminal oxido group to give OH species. The OH group has a crucial role in the condensation steps, because it initiates an attack on a metal center resulting to form a dinuclear complex through OH bridging. The reaction is completed by proton transfer reaction on a terminal OH group, prompting the water elimination reaction to afford O-bridging.