How to change a position of a template ion staying inside a cavity?
- Mechanistic insights into template-driven polyoxovanadate self-assembly: the role of internal and external templates,
S. Repp, K. L. Junginger, D. Sorsche, T. Zorn, A. Pöppler, Y. Kikukawa, Y. Hayashi, and C. Streb, Dalton Trans.,52, 4002-4007 (2023). DOI: 10.1039/D3DT00252G. The synthetic methodology for the hydroxides relevant to the water splitting catalysts was developed
- Synthesis of Water-Soluble Planar Cobalt(II), Nickel(II), and Copper(II) Hydroxo Clusters Using a (1,4,7-Triazacyclononane)cobalt(III) Complex as a Hydrolysis-Terminating Group,
Sugiarto, Y. Imai and Y. Hayashi, Inorg. Chem,62, 1845-1854 (2023). DOI: 10.1021/acs.inorgchem.2c01046. How to make water splitting catalysts better?
- New insights for molecular artificial photosynthesis catalysts by metsl-oxide and/or metal-sulfides clusters,
Y. Hayashi, Sulphuric acid and industry, 75, 115-130 (2022). Keep a lid on and do not let an azide jumping out from the cavity
- Hydrophobic interaction of V12 bowl-type dodecavanadates with alkyl ammonium cations,
H. Iwai, T. Kasamatsu, S. Kuwajima, Y. Kikukawa, and Y. Hayashi, Polyhedron, 224, 115985 (2022). DOI: 10.1016/j.poly.2022.115985. The isolation of a key polyoxovanadate species in an oxidation catalytic cycle
- Synthesis and oxidation catalysis of a difluoride-incorporated polyoxovanadate and isolation of active vanadium alkylperoxo species,
Y. Kikukawa, Y. Sakamoto, H. Hirasawa, Y. Kurimoto, H. Iwai and Y. Hayashi, Catal. Sci. Technol., 12, 2438 (2022). DOI: 10.1039/D1CY02103F. The liberated species from a polyoxovanadate framework in a catalytic oxidation reaction
- Synthesis of cyanooxovanadate and cyanosilylation of ketones,
Y. Kikukawa, H. Kawabata, and Y. Hayashi, RSC Advances, 11, 31688 (2021). DOI: 10.1039/D1RA05879G. A carbanion captured in the vanadium bowl
- Isolation of a Nitromethane Anion in the Calix-Shaped Inorganic Cage,
Y. Kikukawa, H. Kitajima, S. Kuwajima and Y. Hayashi, Molecules, 25, 5670 (2020). DOI: 10.3390/molecules25235670. The diatomic molecule, Br2, fitted in an inorganic bowl, showed IR-stretching band
- Induced Fitting and Polarization of a Bromine Molecule in an Electrophilic Inorganic Molecular Cavity and Its Bromination Reactivity,
Y. Kikukawa, K. Seto, D. Watanabe, H. Kitajima, M. Katayama, S. Yamashita, Y. Inada and Y. Hayashi, Angew. Chem. Int. Ed., 59, 14399-14403 (2020). DOI: 10.1002/anie.202007406. How to stabilize titanium(IV) cation species in water ?
- Stabilization of titanium(IV) and indium(III) complexes by coordination of [MoO3(1,4,7-triazacyclononane)] metalloligand in aqueous solution,
Sugiarto, S. Kazakami, K. Kawamoto and Y. Hayashi, Inorg. Chem. Acta., 509, 119691 (2020). DOI: 10.1016/j.ica.2020.119691. Iron dinuclear complexes with Bifurcated hydrogen bondings reminiscent of the FeMo cofactor
- Artificial bioinorganic clusters of dinuclear 3d-transition metal ions coordinated by an inorganic coordination ligand,
Sugiarto, K. Kawamoto and Y. Hayashi, J. Inorg. Biochem., 201, 110821 (2019). DOI: 10.1016/j.jinorgbio.2019.110821. Double-cubane manganese(III, IV) complexes supported by vanadium oxide ligands
- Redox Active Mixed-Valence Hexamanganese Double-cubane Complexes Supported by Tetravanadates,
T. Maruyama, A. Namekata, H. Sakiyama, Y. Kikukawa and Y. Hayashi, New J. Chem., 43, 17703-17710 (2019). DOI: 10.1039/C9NJ02437A. Estimation of dodecavanadate-host affinity against various organic guest molecules
- Evaluation of the chemo- and shape-selective association of a bowl-type dodecavanadate cage with an electron-rich group,
Y. Kikukawa, H. Kitajima and Y. Hayashi, Dalton Trans., 48, 7138-7143 (2019). DOI: 10.1039/c9dt00462a A yttrium probe for the exploration of rare-earth polyoxovanadates
- Synthesis and Characterization of Yttrium-containing Sandwich-, Ring-, and Cage-type Polyoxovanadates,
T. Maruyama, H. Kawabata, Y. Kikukawa and Y. Hayashi, Eur. J. Inorg. Chem., 3, 529-533 (2019). DOI: 10.1002/ejic.201800540 An inorganic bowl with flipping bottom
- Solid-State Umbrella-type Inversion of a VO5 Square-Pyramidal Unit in a Bowl-type Dodecavanadate Induced by Insertion and Elimination of a Guest Molecule,
Y. Kikukawa, K. Seto, S. Uchida, S. Kuwajima and Y. Hayashi, Angew. Chem. Int. Ed., 57, 16051-16055 (2018). DOI: 10.1002/anie.201809120 A tube polyoxovanadate composed of stacking V7 rings
- Synthesis and structural characterization of tube type tetradecavanadates,
S. Kuwajima, Y. Arai, H. Kitajima, Y. Kikukawa and Y. Hayashi, Acta Cryst., C47, 1295-1299 (2018). DOI: 10.1107/S2053229618008914 A water soluble divanadate dimer resembling the vanadium-pentoxide structure
- Strategic Isolation of a Polyoxocation Mimicking Vanadium(V) Oxide Layered-Structure by Stacking of [H2V2O8]4− Anions Bridged by (1,4,7-Triazacyclononane)Co(III) Complexes,
Sugiarto, K. Kawamoto and Y. Hayashi, Front. Chem., 6:375, (2018). DOI: 10.3389/fchem.2018.00375 Polyoxometalates turned into polyoxocations
- Synthesis of cationic molybdenum-cobalt heterometallic clusters protected against hydrolysis by macrocyclic triazacyclononane complexes,
Sugiarto, T. Tagami, K. Kawamoto and Y. Hayashi, Dalton Trans., 47, 9657-9664, (2018). DOI: 10.1039/C8DT01226A Nuclearity control in a decavanadate ligand
- A Highly-flexible Cyclic-decavanadate Ligand for Interconversion of Dinuclear- and Trinuclear-cobalt(II) and Manganese(II) Cores,
T. Maruyama, Y. Kikukawa, H. Sakiyama, M. Katayama, Y. Inada, Y. Hayashi, RSC. Adv., 7, 37666-37674, (2017). DOI: 10.1039/c7ra05941h A Pd-supported polyoxovanadate
- Synthesis and Characterization of a Palladium-Supported Fluoride-Incorporated Dodecavanadate,
K. Miftahul, Y. Kikukawa, Y. Hayashi, Chem. Lett., 46, 1406-1408, (2017). DOI:10.1246/cl.170594 An inorganic-squeeze-bowl
- Small Molecular Anion Recognition by a Shape-responsive Bowl-type Dodecavanadate,
S. Kuwajima, Y. Kikukawa, Y. Hayashi, Chem. Asian J., 12, 1909-1914, (2017). DOI: 10.1002/asia.201700489 Dodecavanadate bowls revisited
- A Bowl-Type Dodecavanadate as a Halide Receptor,
S. Kuwajima, Y. Ikinobu, D. Watanabe, Y. Kikukawa, Y. Hayashi, and A. Yagasaki, ACS Omega, 2, 268-275, (2017). DOI: 10.1021/acsomega.6b00408 How close can you force two anions together ?
- Synthesis and Structural Characterization of Trimanganese-Containing Polyoxovanadates with Carboxylate Ligands,
T. Maruyama, Y. Kikukawa, K. Kawamoto, and Y. Hayashi, Eur. J. Inorg. Chem., 3, 596-599, (2017). DOI:10.1002/ejic.201601274 Electroabsorption (Stark) Spectra of Transition Metal Complexes,
K. Kawamoto, H. Hashimoto, Bull. Jpn. Soc. Coord. Chem., 67, 75-79, (2016). DOI: 10.4019/bjscc.67.75Play a catch by a molecular mitt
- A chloride capturing system via proton-induced structure transformation between opened- and closed-forms of dodecavanadates,
Y. Inoue, Y. Kikukawa, S. Kuwajima and Y. Hayashi, Dalton Trans., 45, 7563-7569, (2016). DOI:10.1039/C6DT00963H Deca-, dodeca-, and tridecavanadates
- Structure Transformation among Deca-, Dodeca- and Tridecavanadates and Their Properties for Thioanisole Oxidation,
Y. Kikukawa, K. Ogihara, Y. Hayashi, Inorganics, 3, 295-308, (2015). DOI:10.3390/inorganics3020295 Polyoxovanadate review 2
- Coordination Chemistry of Polyoxovanadates as Inorganic Ligands,
Y. Hayashi, Bull. Jpn. Soc. Coord. Chem., 66, 12-25, (2015). DOI:10.4019/bjscc.66.12 Fluoride-incorporated polyoxovanadates
- Synthesis and Characterization of Fluoride-incorporated Polyoxovanadates,
Y. Kikukawa, T. Yokoyama, S. Kashio, Y. Hayashi, J. Inorg. Biochem., 147, 221-226 , (2015). DOI:10.1016/j.jinorgbio.2015.02.010 Disk, Bowl, to Ball
- Structural Conversion from Bowl- to Ball-type Polyoxovanadates: Synthesis of a Spherical Tetradecavanadate through a Chloride-incorporated Bowl-type Dodecavanadate,
T. Kobayashi, S. Kuwajima, T. Kurata, Y. Hayashi, Inorg. Chim. Acta., 420, 69-74, (2014). DOI:10.1016/j.ica.2014.03.035 Descrete hexadecavanadates
- Discrete Spherical Hexadecavanadates Incorporating a Bromide with Oxidative Bromination Activity,
N. Kato and Y. Hayashi, Dalton Trans., 42, 11804-11811 (2013). DOI:10.1039/C3DT50521A Polyoxometalate-based Frameworks with a Linker of Paddlewheel Diruthenium(II, III) Complexes,
A. Hashikawa, Y. Sawada, Y. Yamamoto, M. Nishio, W. Kosaka, Y. Hayashi, H. Miyasaka, Cryst. Eng. Comm., 15, 4852-4859 (2013). DOI: 10.1039/C3CE40426ABig cations in polyoxovanadate rings
- Early-Lanthanide Complexes with All-Inorganic Macrocyclic Polyoxovanadate Ligands,
M. Nishio, S. Inami, Y. Hayashi, Eur. J. Inorg. Chem., 10, 1876–1881 (2013). DOI:10.1002/ejic.201201435 Coulombic Aggregations of MnIII salen-Type Complexes and Keggin-Type Polyoxometaltes: Isolation of Mn2 Single-Molecule Magnets,
Y. Sawada, W. Kosaka, Y. Hayashi, H. Miyasaka, Inorg. Chem., 51, 4824–4832 (2012). DOI: 10.1021/ic300215qInorganic Frameworks Made by Combining Paddle-wheel Diruthenium(II, III) Complexes and Polyoxometalate Clusters,
Y. Sawada, Y. Yamamoto, M. Nishio, W. Kosaka, Y. Hayashi, H. Miyasaka, Chem. Lett., 41, 212–214 (2012). DOI: 10.1246/cl.2012.212Lanthanide complexes with polyoxovanadate ligands
- Lanthanide Complexes of Macrocyclic Polyoxovanadates: Synthesis, Characterization, and Structure Elucidation by X-ray Crystallography and EXAFS Spectroscopy, M. Nishio, S. Inami, M. Katayama, K. Ozutsumi, Y. Hayashi, Inorg. Chem., 51, 784–793 (2012). DOI:10.1021/ic200638f
Reduced hexa-lacunary Dawson complexes
- Isolation of a Stable Lacunary Dawson-type Polyoxomolybdate Cluster,
A. Hashikawa, M. Fujimoto, Y. Hayashi, H. Miyasaka, Chem. Commun., 47, 12361–12363 (2011). DOI:10.1039/C1CC15439G Polyoxovanadate Review
- Hetero and Lacunary Polyoxovanadate Chemistry: Synthesis, Reactivity and Structural Aspects, Y. Hayashi, Coord. Chem. Rev., 255, 2270–2280 (2011). DOI:10.1016/j.ccr.2011.02.013
An Anion Trap Inside an Anion
- Synthesis and Characterization of Chloride-incorporated Dodecavanadate from Dicopper Complex of Macrocyclic Octadecavanadate, T. Kurata, Y. Hayashi, K. Isobe, Chem. Lett., 708–709 (2010). DOI:10.1246/cl.2010.708
All-inorganic Dimanganese and Dicobalt Complexes in Vanadate Macrocycle Ligands
- Dinuclear Manganese and Cobalt Complexes with Cyclic Polyoxovanadate Ligands: Synthesis and Characterization of [Mn2V10O30]6– and [Co2(H2O)2V10O30]6–, S. Inami, M. Nishio, Y. Hayashi, K. Isobe, H. Kameda, T. Shimoda, Eur. J. Inorg. Chem., 34, 5253–5258 (2009). DOI: 10.1002/ejic.200900609
Lacunary Polyoxovanadates with a Guest Anion.
- Formation of V(V) Spherical Polyoxovanadates and Interconversion Reactions of Dodecavanadate Species, K. Okaya, T. Kobayashi, Y. Koyama, Y. Hayashi, K. Isobe, Eur. J. Inorg. Chem., 34, 5156–5163 (2009). DOI: 10.1002/ejic.200900605
A Td unit on a tetravanadate ring.
- Tetrahedral Cobalt(II) and Zinc(II) Chloride with Tetravanadate through a Tripod Coordination Mode, T. Kurata, Y. Hayashi, K. Isobe, Chem. Lett., 218–219 (2009). DOI:10.1246/cl.2009.218
Hexamethoxohexavanadate.
- Synthesis of a Bowl-type Dodecavanadate by the Coupling Reaction of Alkoxohexavanadate and Discovery of a Chiral Octadecavanadate, K. Domae, D. Uchimura, Y. Koyama, S. Inami, Y. Hayashi, K. Isobe, H. Kameda, T. Shimoda, Pure. Appl. Chem., 81, 1323–1330 (2009). DOI:10.1351/PAC-CON-08-08-25
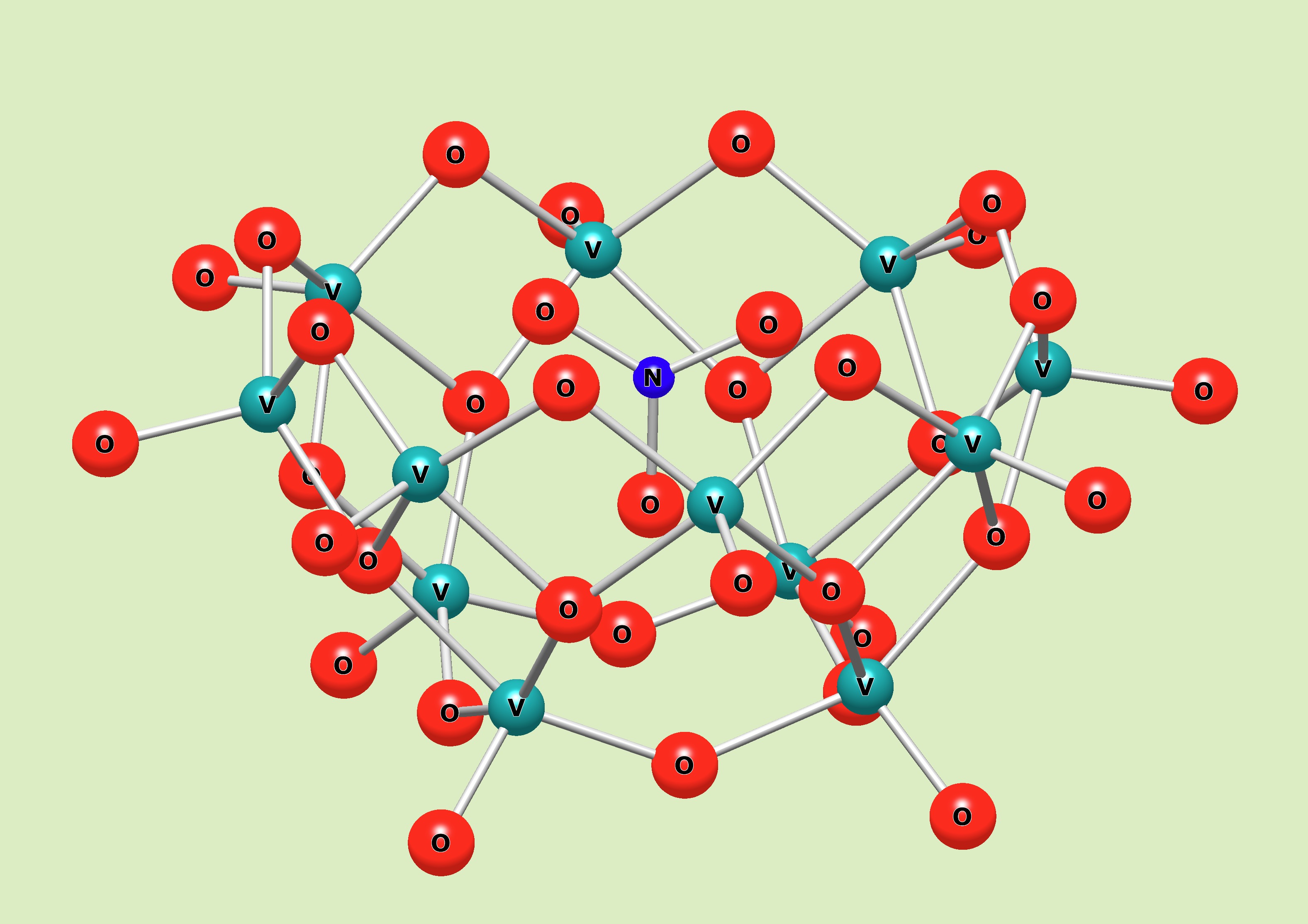
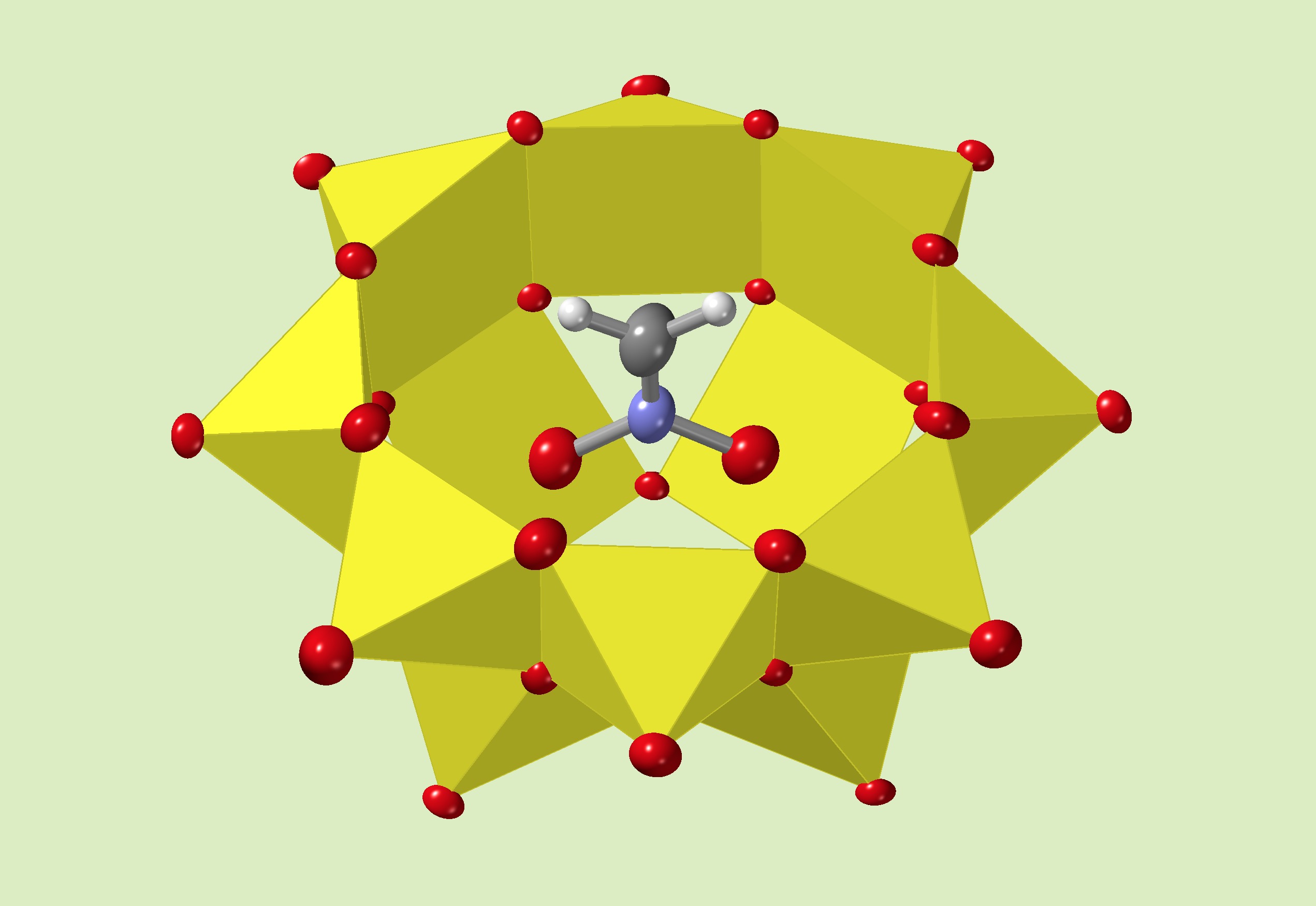
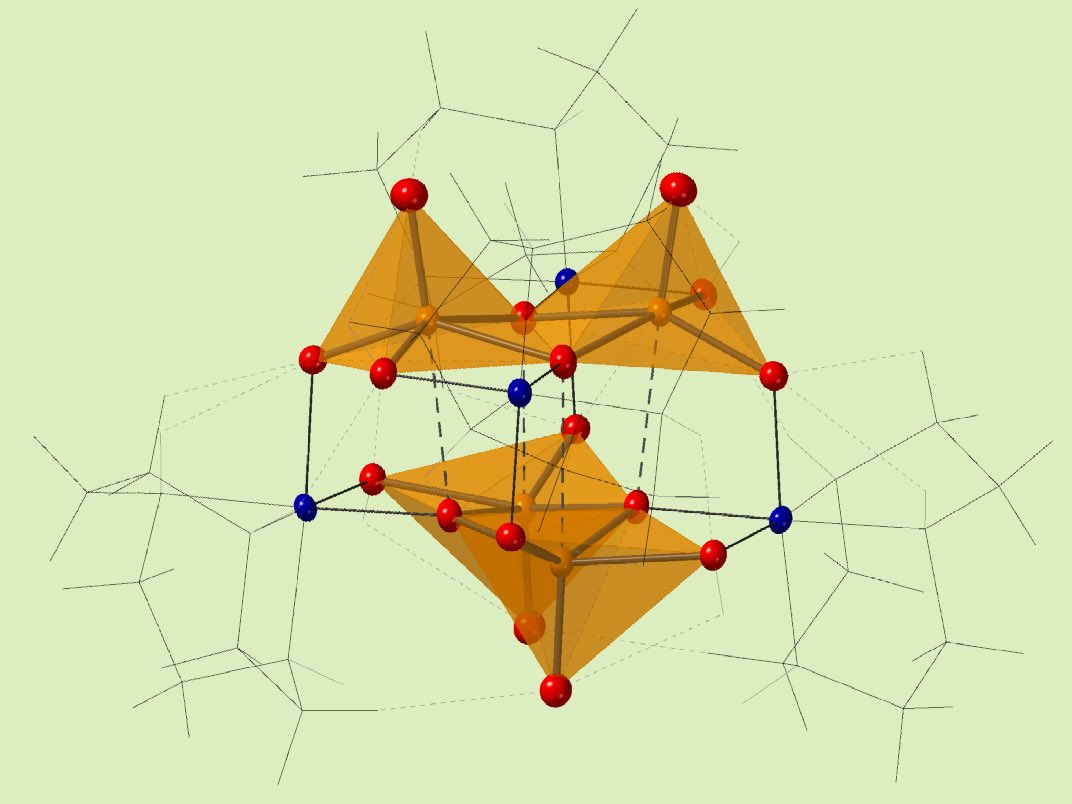
The Streb group from Mainz and Ulm have established a chemical manipulation of a template position in the cavity of the V12 inorganic bowl molecule by controlling a charge from a counter cation. They choose several counter cations best fitted on the entrance of the bowl molecule, and enable to attract the template ion such as nitrate, chloride, bromide. It drives the template move out from the stable position in the cavity. Dr. Repp investigated a part of his work in Kanazawa University.
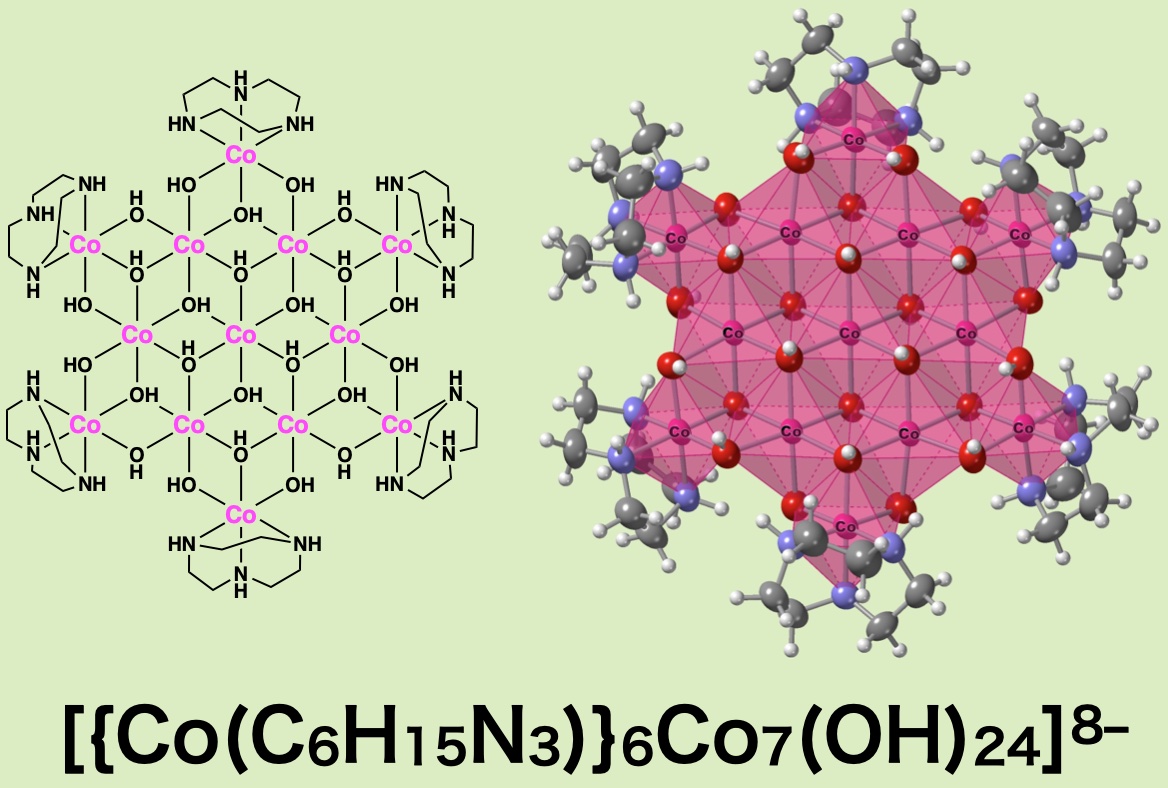
To improve water splitting catalysis firther, the molecular level nano-size manipulation of the structure of oxides and hydroxides are required. The proposed water splitting catalysis in situ or on the surface of the electrodes, the planer cobalt hydroxide clusters, is an hypothesized active species. There are no accessible synthetic method for the water soluble hydroxide species because of the labile rapid formation and transformatin of those species. To terminate the water-elimination condensation process, we employ chemical protection group by using a coordination complex of an inert Co(III) complex. In this paper, how to isolate a various multinuclear planer-hydroxide ions of Co(II), Ni(II) and Cu(II) was explained and the stability of those frameworks were confirmed through XAFS study in water.
The era of artificial photosynthesis, likely to arrive in the near future, promises more efficacy. The idea is not new, but a significant improvement has to be made due to the fact that all targeted materials are readily available from fossil fuel. The choice of a suitable catalyst is a key to a successful uphill reaction. Because oxides and sulfides change its structure, an intricate design of materials that make the catalyst more lasting and trouble free is necessary. The molecular chemistry of a pure material with a consistent knowledge of not only a composition but also a nano-structure is requested. In this manuscript, the basic principles of inorganic chemistry behind oxides and sulfides are explained and the recent development of vanadium oxide clusters as a model oxide-cluster molecule including a vanadium-oxide layer copmlex, a hetero vanadium-oxide cluster, a vanadium-oxide host molecule, a cubane-type manganese vanadium-oxide cluster mimicking the structure of the photosynthesis active center, and a planer-type cobalt- and a nickel-hydroxide cluster that is the proposed active center for artificial photosynthesis, are reported in Japanese language.

Dodecavanadate hosts can incorporate a small guest anion like an azide. In a solution, the linear azide sticked out from the cavity. The addition of tetrabutyl ammonium salts lead to the azide orientation in a different orientation. It acts as a lid to make the azide confined in an elongated cavity fitted to the linear azide shape. 51V NMR spectra clealy distinguish a vertical or a holizontal capturing of the azide, with or without the lid, reflected by the different orientations accompanying the elongation of the host framework. The lid effect by t-butyl ammonium cation, was discovered for the inorganic oxide bowl host.

Organometallic chemists have been performed to elucidate a catalytic mechanism through an isolation of the proposed species in their catalytic cycle. In polyoxometalate science, the confirmation of a chemical species by an isolation involved in a catalytic cycle is also important but no example is yet to appear. In this paper, we have accomplished a sucessful isolation of a series of polyoxovanadates involved in an epoxidation and a bromination catalytic reaction of alkenes: a precursor species, a peroxo species, and an alkoxo species on a V12 polyoxovanadate framework. All the key species in the proposed catalytic cycle have been characterized, and the progress of the reaction in situ was elucidated through 51V NMR observation and the mechanism was rationalized based on the isolated species.
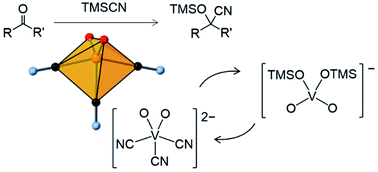
When a catalytic oxidation reaction is carried out by using polyoxometalates, a monomeric complex may be liberated from the main framework and acts as a catalytic active species. In this paper, we investigated various cyanocilylation reaction for ketones, and revealed the dissociation of [VO2(CN)3]2- from the main framework. It was sucessfully isolated and characterized along with other active species. The proposed mechanism was verified by monitoring the reaction progress by 51V NMR in situ.
The inorganic host, V12-type vanadium bowl, provides a unique cavity by the arrangement of twelve vanadium atoms. The ceramic oxide cage can stablize a tentative intermediate species, especially an anionic intermediate: (NO2CH2)-. In this report, a nitronate-incorporated V12 host was elucidated and fully characterized. The absence of proton from a nitromethane guest was confirmed by X-ray crystallographically as well as 1H or 13C NMR and mass spectroscopy. The side reaction in nitromethane solvent may be effectively blocked by an addition of V12 host which catches a nitronate anion.
The V12-type vanadium polyoxometalates are suited to store a noxious oxidant such as Br2 as a guest, and stabilized. It make the toxic gas solid, no longer to be a hazardous vapor. The V12 host is an inorganic ceramic bowl in a molecular size. The size of Br atom allows the best fits in the cavity. The resulting incorporation mode of diatomic Br2 is explained by one Br atom sitting in the cavity and the other Br atom sticking out from the cavity. The diatomic Br2 molecule is IR-forbidden and no IR peak is supposed to observe, however, when Br2 sits on the geometrically asymmetric environment, there is a possibility of observing an IR stretching band. In our case, we believe a Br2 stretching band was observed, first time. The inside of the V12 cavity has the strong cationic environment surrounded by twelve V5+ cations. The positive electric field in the cavity induced the dipolar-polarization of Br2: one Br atom in the cavity forced to polarize to negative, and the other Br atom to positive, resulting an orientation polarization of Br2, and it becomes to be an asymmetric Br2 molecule. By using the exposed Br+ that has a cationic character, alkane oxidation was also demonstrated in a solid state.
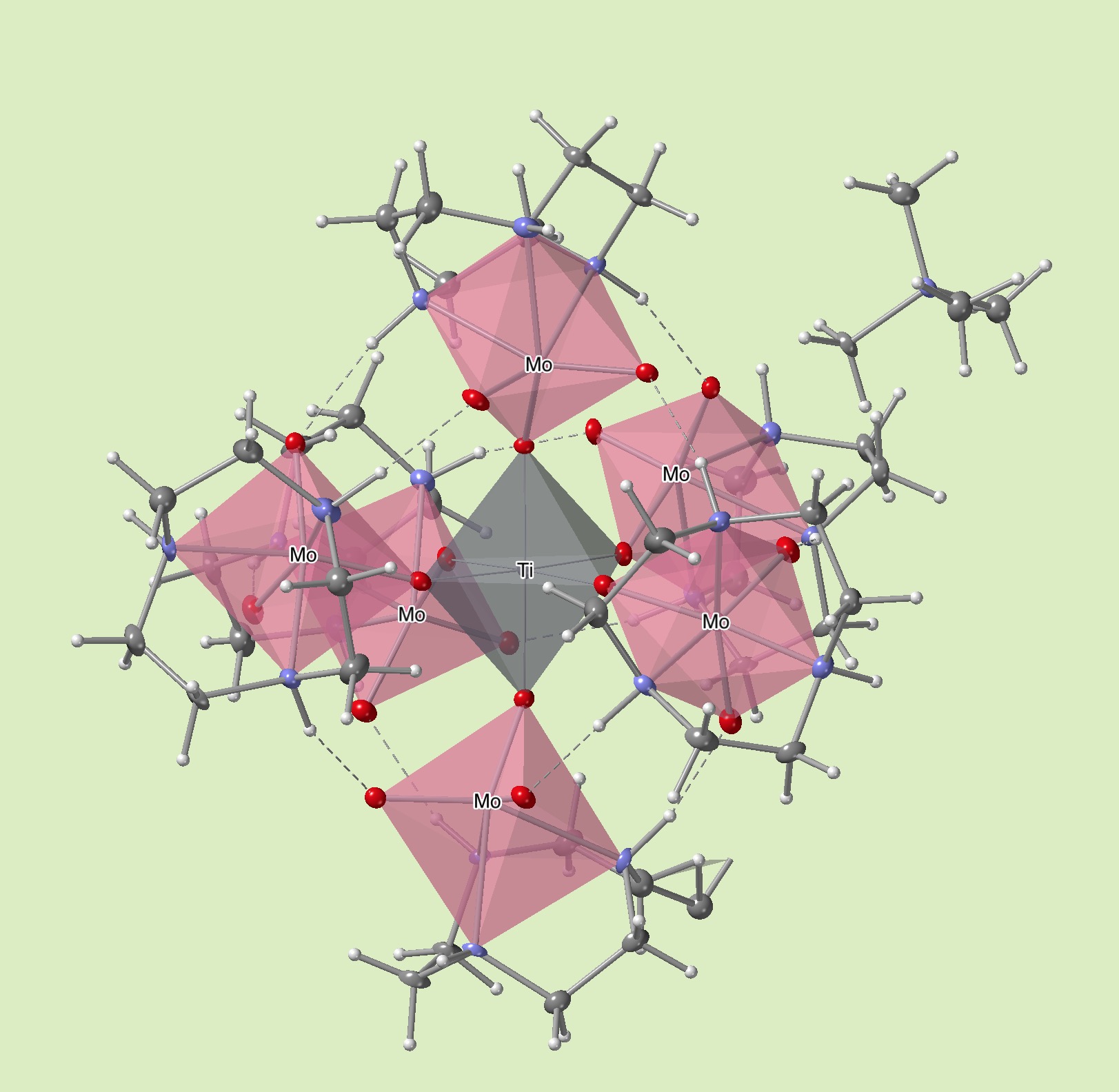
In water, transition metal cations are suceptible to hydrolysis or polymerization by water elimination condensation reaction giving hydroxides or oxide preipitates. This relatively rapid process is problematic in inorganic synthesis for a targetted design of a hydroxo/oxo-cluster species. For example, titanium or aluminum cations are known to give immidiate precipitation products that is a mixture of polymerized species without a chance of ordering. We have developed a new methodology by temporarily blocking the condensation reaction using a chemical protecting group. This is our agenda that any transition metal cations can be chemically protected against hydrolysis by using our inorganic ligand as a protecting group. In this paper, we have demonstrated the utilization of the inorganic ligand to stabilize Ti(IV) and In(III) (In(III) species behaves similar to Al(III)) in water.
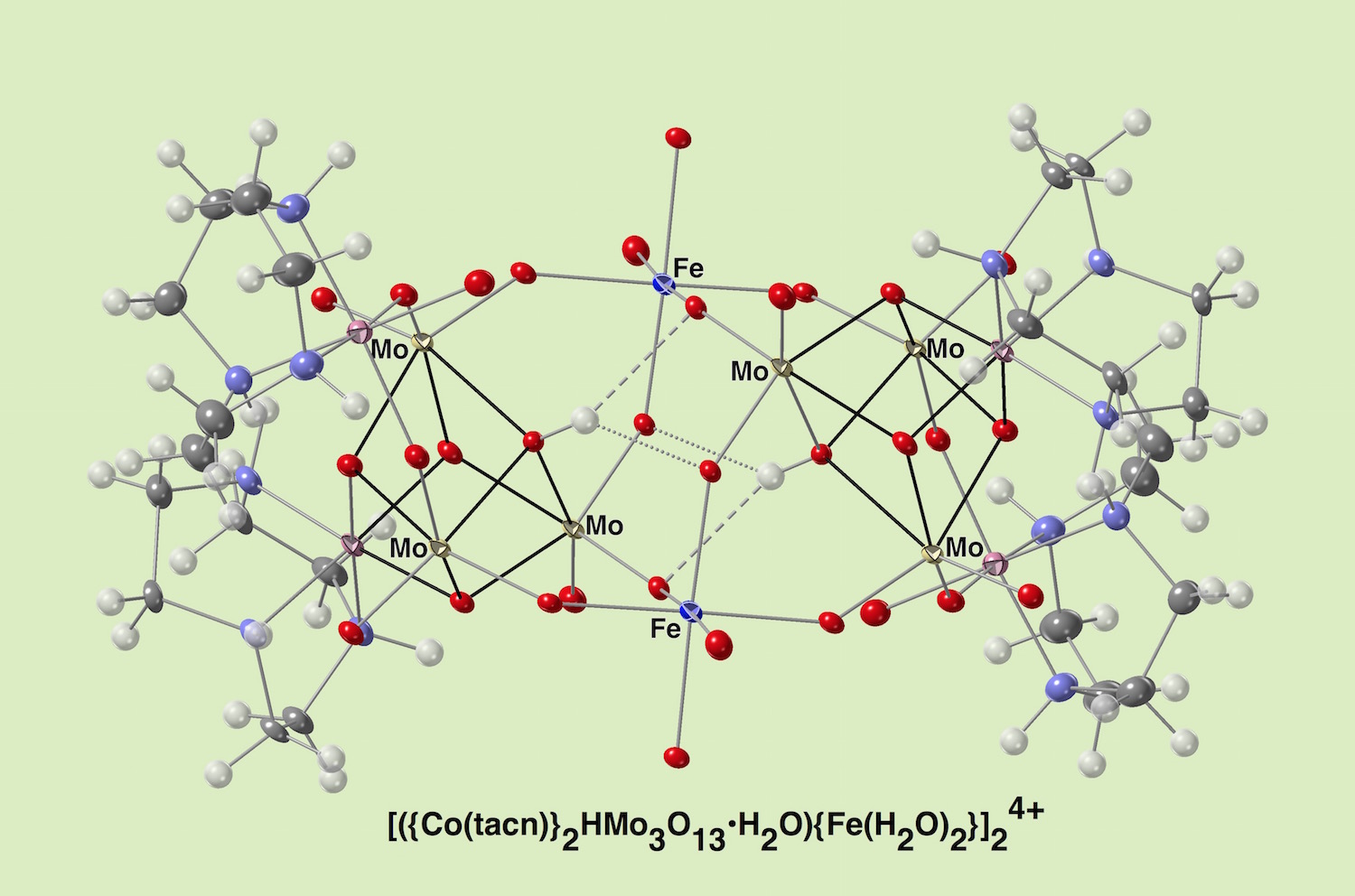
We have designed a new artificial [CoMo3O3(OH)] ligand that is able to support a 3d-transition-metal dinuclear unit. It provides two sets of cis-oxido donors to occupy a coordination sphere on a 3d-transition ion. The dinuclear cores were separated a relatively long distance in the range of 5.4-5.8 Å, and it showed an electric communication in a redox-process mediated by bifurcated hydrogen bondings shown in a dottted line on µ3-O oxygen atoms through the redox-active cubane-type ligands. The dinuclear unit was wrapped by hydrogen-bonding networks and it made the cluster soluble in water. This study suggests a new way of investigating the role of biologically relevant cubane-clusters. The electronic interaction path through cubane-cores is interesting in terms of constructing a redox active scaffold that is reminiscent of the FeMo cofactor.
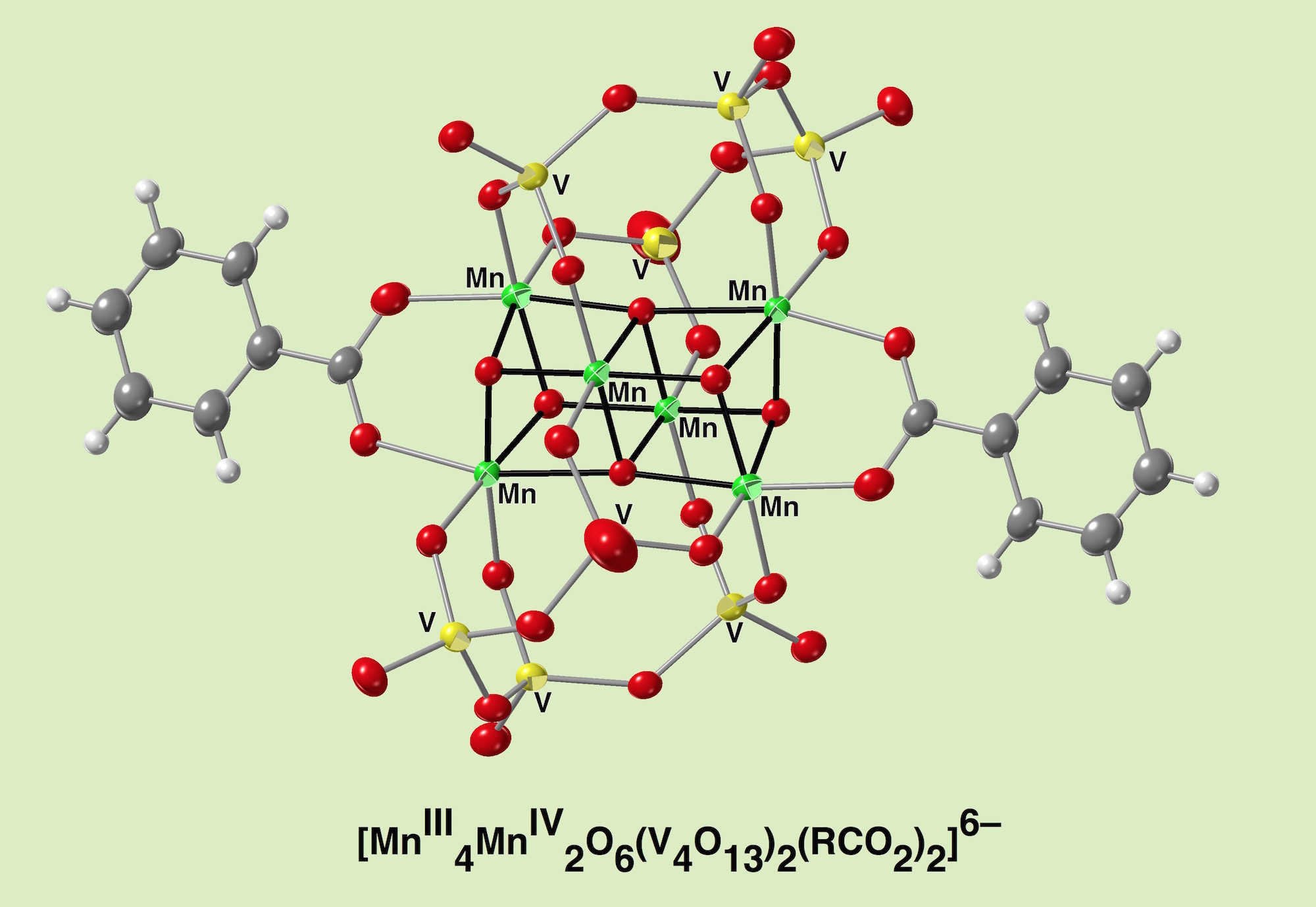
A hexamanganese {Mn(III)4Mn(IV)2O6} unit, a relevant structure to the water oxidation center in photosystem II, is stabilized in a VO4-based cyclic ligand that is robust against oxidation reaction because of its highest oxidation state of V(V). It is feasibile to dissolve in organic solvents. The synthetic procedure was the insertion of two manganese ions at two vacant sites in a binuclear manganese complex, [Mn2(V5O15)2]6–, followed by the substitution of a VO4 group with a manganese ion in the pentameric vanadate ring, achieving a double-cubane-type Mn6O6 core that was coordinated by polyoxovanadates and benzoate groups. It is redox active showing two sets of reversible waves in electrochemistry. The manipulation of magnetic interaction patterns among manganese atoms was achieved by using the effect of a substituent on benzoates as well as an oxidation state of the manganese core. The system presented here achieved the construction of a double-cubane manganese unit supported by oxidatively-robust polyoxovanadates, and provides the framework of future work to assess the performance of oxidative catalytic properties.

We have examined the affinity of the V12 inorganic host with flipping bottom against various organic molecules. It showed a different affinity against small organic molecules: a molecule with a polar functional group, organic-halides, cyclic-carbonates, lactones, ketones, and aldehydes, gives a different affinity. The quantitative analysis of the host-guest property was investigated through 51V NMR study. Among organic halide molecules, bromide derivatives showed the highest affinity. The guest-free bowl has one VO5 unit flipped as if it filled the space at the cavity. When the guest-free bowl accepts an appropriate guest, the flipped VO5 unit rearranges in a way that the terminal oxido-group pointing outside the cage as if letting the cavity open, and this structure isomerization is an important characteristic of the vanadium-oxide bowls.
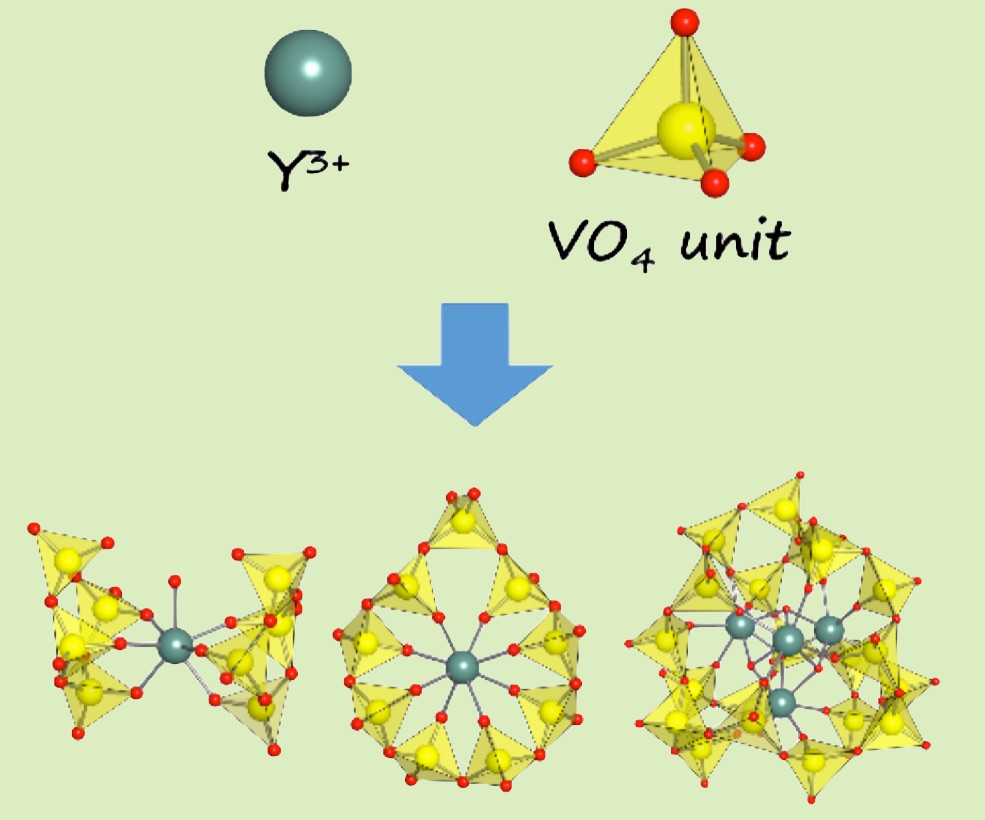
To understand the solution chemistry of rare-earth polyoxovanadates, we have investigated yttrium polyoxovanadate speciation in various experimental conditions in a different molar ratio, solvents, and under a presence of additives. The diamagnetic nature of Y(III) allows us a direct monitoring of a solution species through 51V NMR. As a result, we demonstrate how to manipulate rare-earth polyoxovanadates in three different types of vanadate-frameworks among sandwich-, ring- and cage-forms. A new V20-cage is able to hold a luminescent rare-earth cubane core, Ln4(OH)4, in an admanthane-like polyoxovanadate cage.
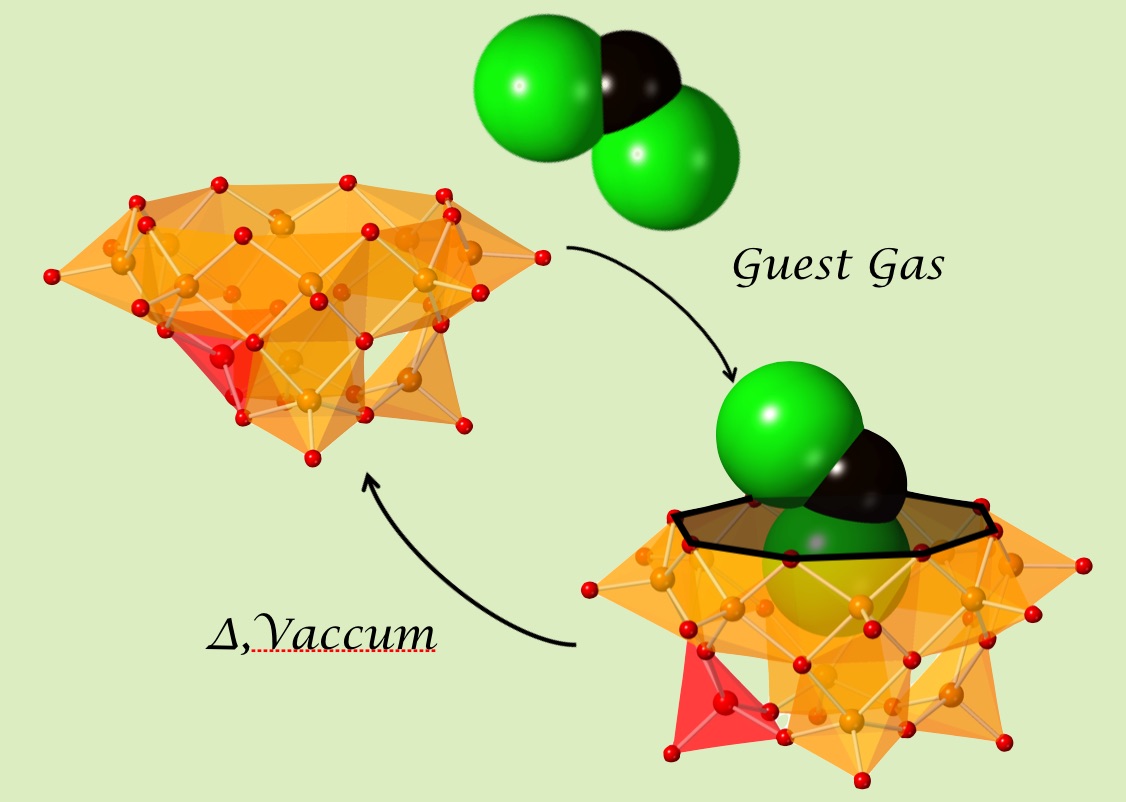
Inorganic host molecules have a flexibe framework. A vanadium-oxide bowl, dodecavanadates(V12) exists with a lid molecule on top of the bowl in a solid state. We created an empty V12 by evaporating the volatile lid, dichrolomethane. But is nature allowing us to make a vacuum space in the bowl? Answere is, of course, No! Instead of a vacuum space, one of a VO5 unit at the bottom flipped upside down, and filled the inner-cavity with its oxygen atom on a pyramid. This rearrangement is reversible and it was observed for the first time in polyoxometalate chemistry. Contacting with a gas molecule, triggered the cavity open by the reverse-flipping, and it makes ready to accomodate the guest, then captures the guest, and the evaporation makes the cavity close again. In this way, the V12 bowl can sense various gas molecules in a solid state.
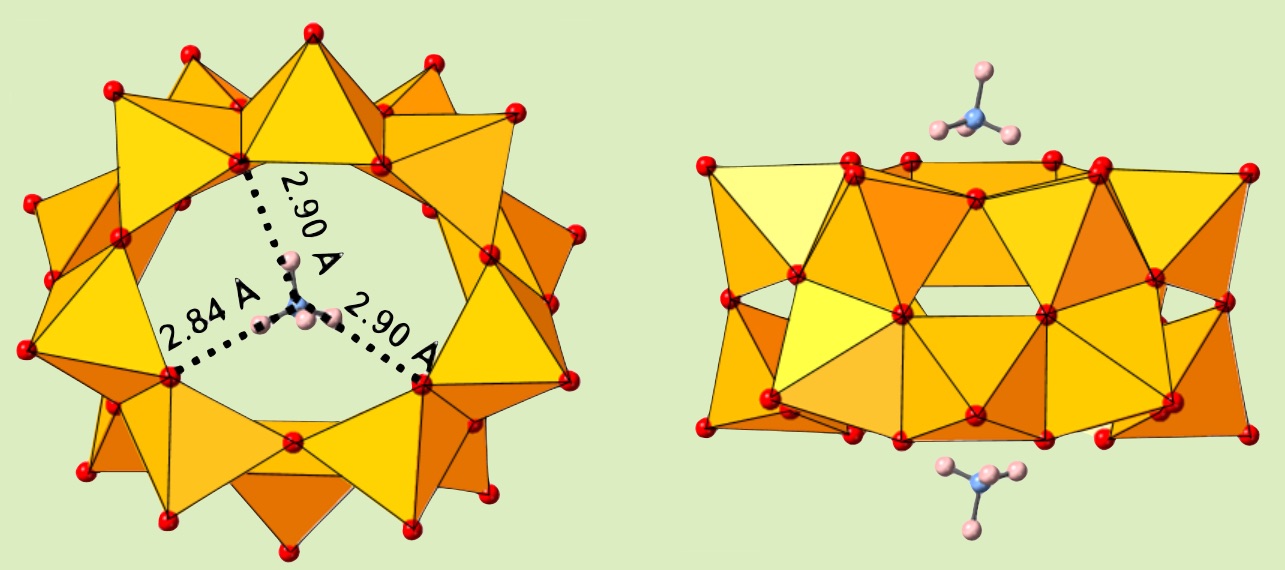
We are studying a formation mechanism of a polyoxovanadate-ring that it fits itself to the size of a guest. In this paper, we have tried to synthesize a tube-type polyoxovanadate from a bowl-shape dodecavandate. After encapsulating a fat guest in the bowl, a stimulus from ammonium ion facilitates the transformation of the bowl into a tube through a formation of hydrogen bonds at both edges. The hydrogen bonds from two ammonium cation at the bottom and the top is a key factor to stabilize the tube structure.
Vanadium(V) oxides (V2O5) have a structure similar to the graphite layer structure. In the oxides, alternatively arranged VO5-based sheets are stacked in layers. To simulate the oxide-sheet structure by a molecule, we have isolated a dimer of vanadate dimers that is a fundamental sheet unit packed in one molecule. It is also regarded as an elongated vanadium cube supported by four cobalt complexes in a windmill arrangement. The layered structure is integrated in a cationic framework reinforced by hydrogen bondings from the peripheral inorganic ligands. The hydrogen bonds help to stabilize the unique stacked structure in a pH range from 2 to 9.
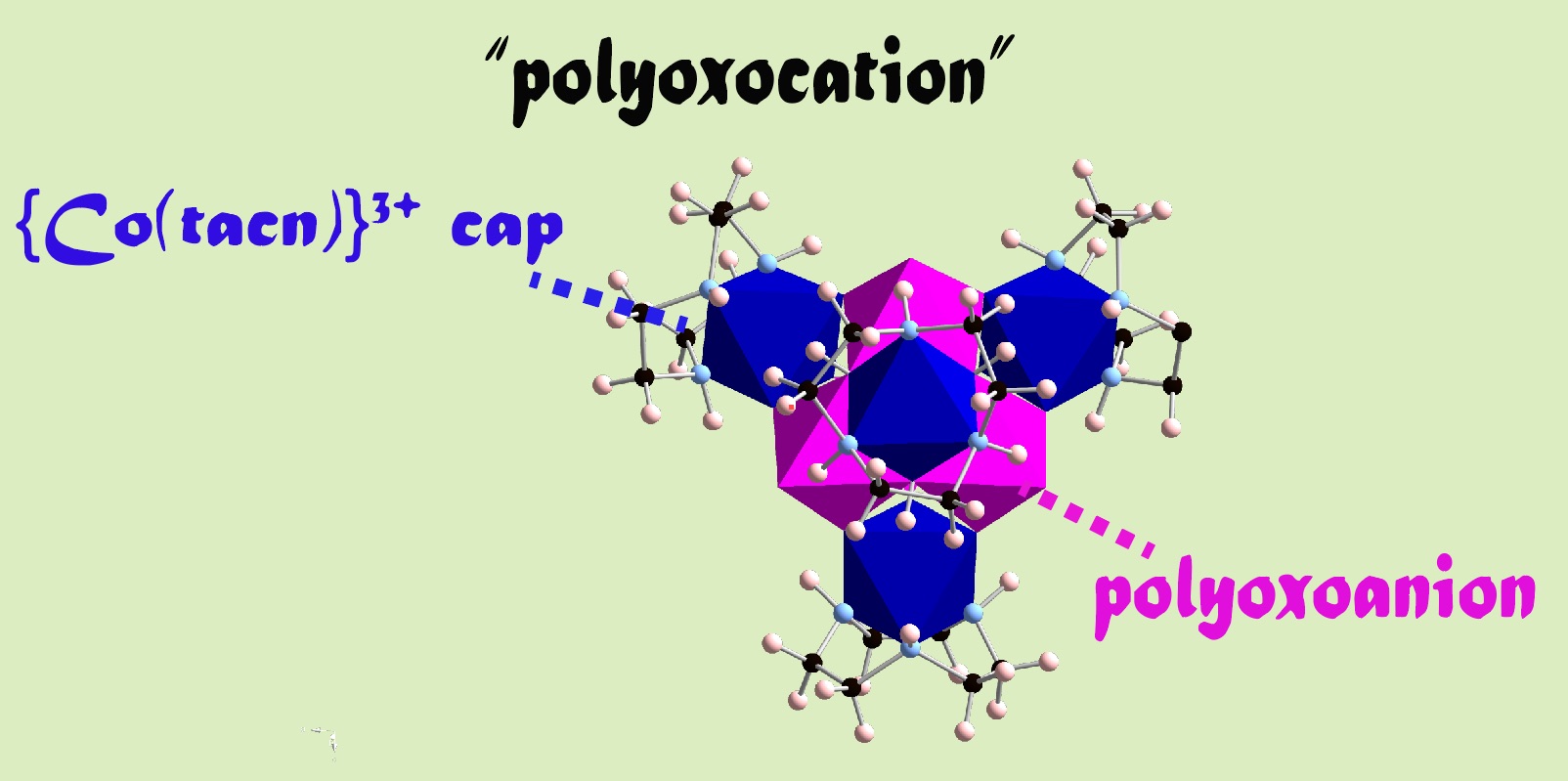
Molybdates form various polyoxometalates which represent a molecular entity of metal oxides. In such anions, no structure contains a metal atom with three terminal oxygen atoms: a consequence of strong trans-influence from M=O multiple bonds. Thus, it is a structural limitation in polyoxometalate chemistry known as the Lipscomb's law. We eliminate the restriction by introducing a functional group. By arranging an exceeding number of cations on the surface of a polyoxometalate, it may turn into polyoxocations; a new type of oxide mimicking molecular frameworks comes to be possible. It also provides hydrogen bonding network to hold the structure in aqueous solution, just like a structure supporting mechanism in enzyme chemistry.
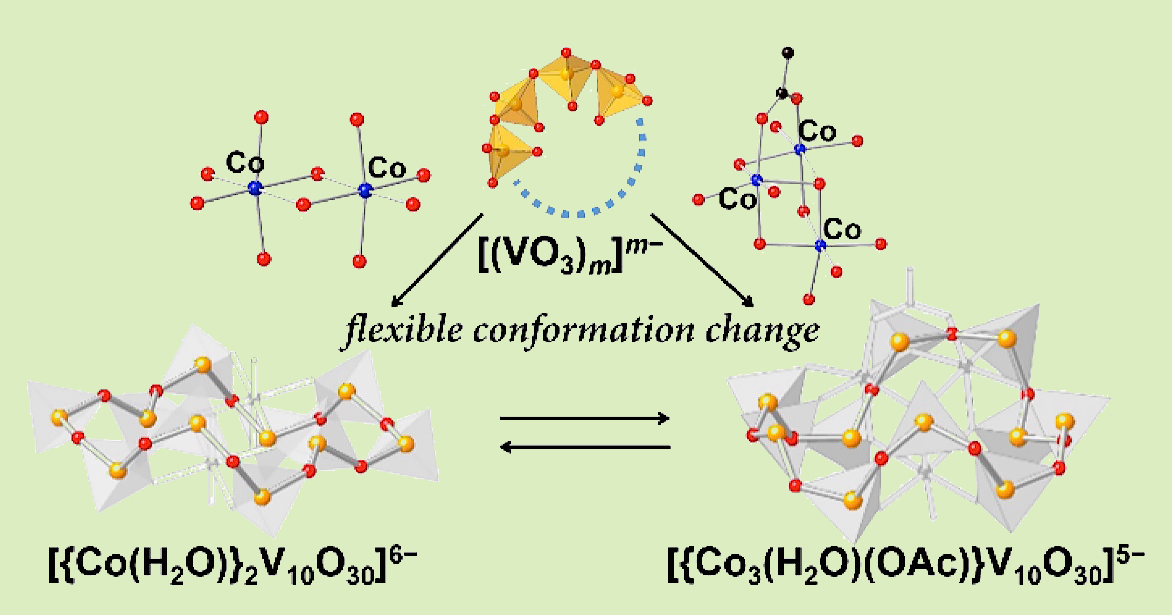
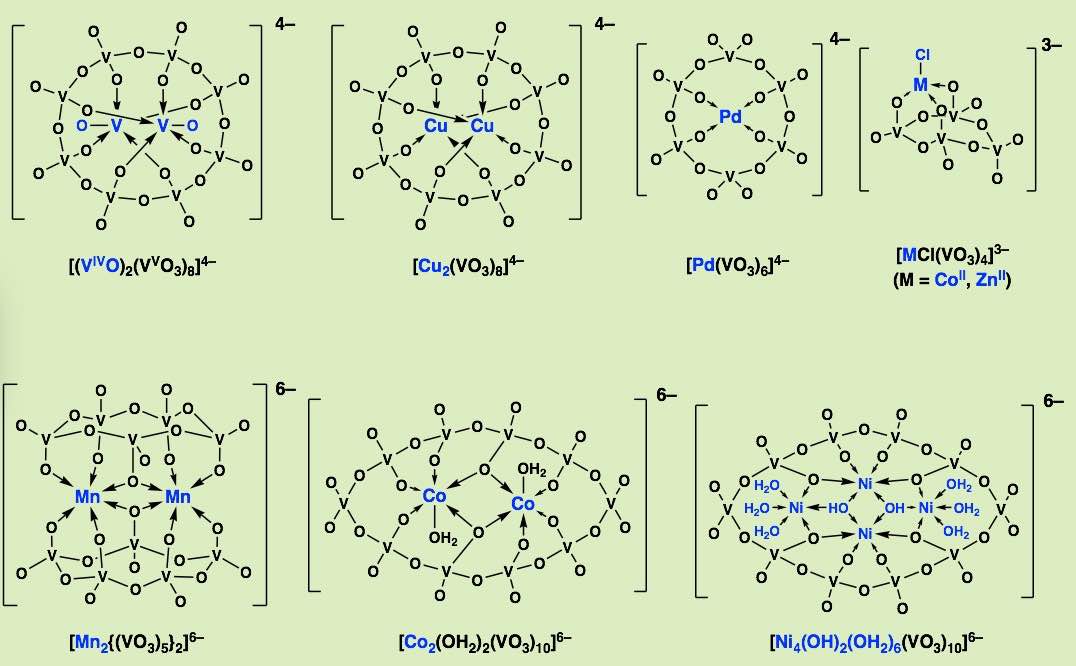
A flexible inorganic polyoxovanadate ligand is able to support a multinuclear core by altering the coordination mode. Unlike organic ligands where a tailor-made design such as an elaborated synthesis, of a binuclear ligand for a binuclear complex, is necessary. Our inorganic ligands allow coordination mode transfomation by offering a switch of an appropriate number of donors and coordination geometry for a dinuclear or a trinuclear cluster unit, without re-designing the ligand, and without multi-step synthetic procedures.
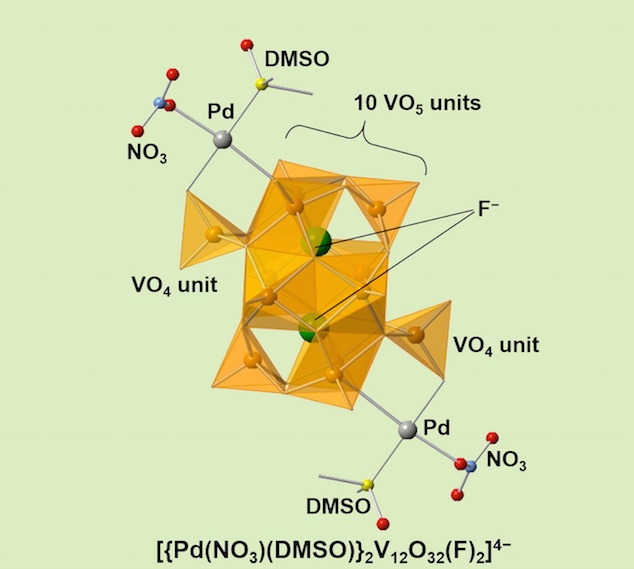
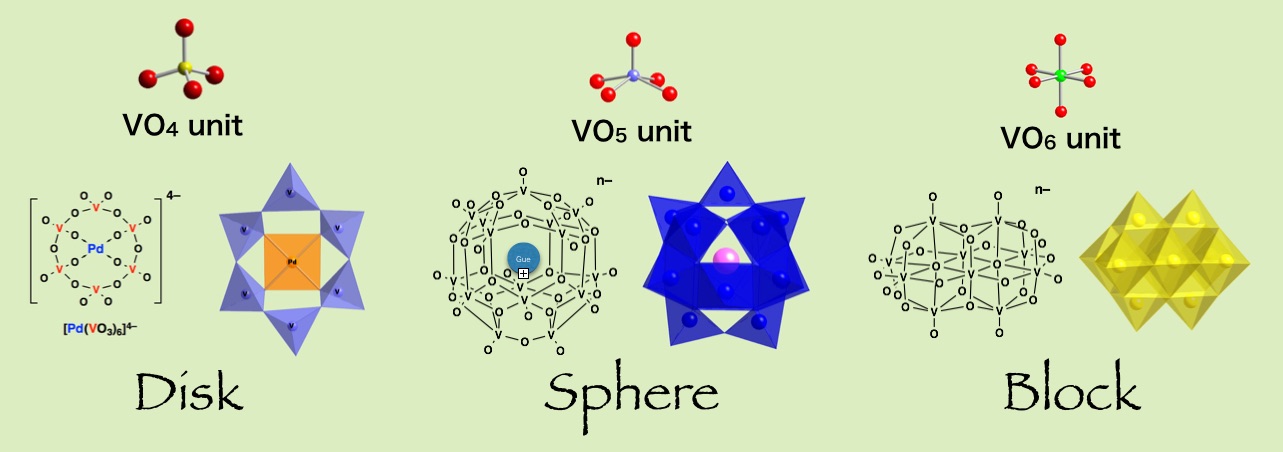
Fluoro-polyoxovanadate frameworks now functioned with two Pd complexes installed on both ends of dodecavanadates. The all-V(V) framework makes vanadium NMR indespensable tool. It has available coordination sites on Pd(II) and more importantly it is soluble in acetonitrile as a discrete molecule and it is diamagnetic, with a potential application in MOF and supramolecuar chemistry as a cross-linker unit. The unique structure is showcasing various inorganic coordination modes, most of it: square-planer, square-pyramid, octahedral, and tetrahedral coordination modes in one molecule.
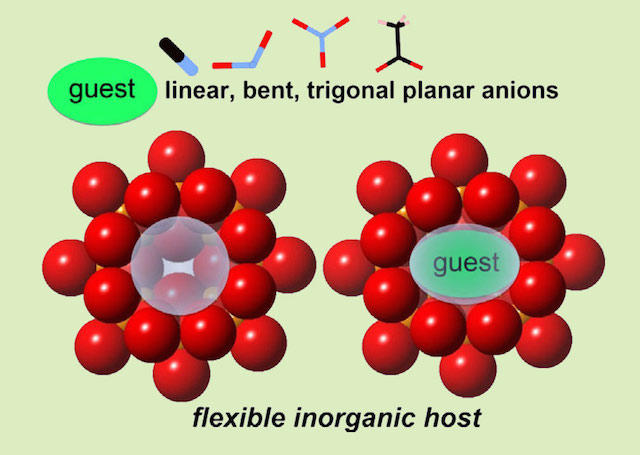
Catch a guest anion of the size even larger than the entrance of an anionic bowl, by squeezing it! It is flexible and is able to capture various multi-atomic anions. Surprisingly, the capturing rates of some big anions are greater than the capturing of small halide ions, because the spreading of partial charges on multiatomic anions may reduce the anionic repulsion between the host and the guest. The ionic host allows an alternation of bond lengths and angles while keeping the over-all geometry the same, it is a shape responsive bowl.
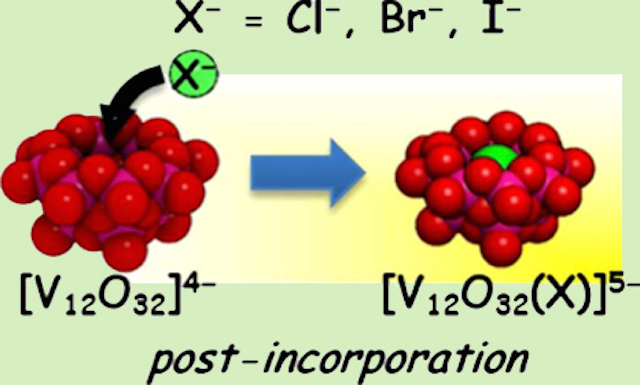
A bowl-type vanadium-oxide molecule has a net charge of -4, yet the inner cavity is positive by the arrangement of twelve vanadium(5+) atoms. When halide is added, a host-guest complex is formed by overcoming a repulsive interaction between -1 from haide and -4 from the bowl. The anion guest sits at the center of the cavity and do not dissociate once it is trapped. "An anion inside an anion trap" comes to be possible becasue of the unique arrangement of the vanadium-cage.
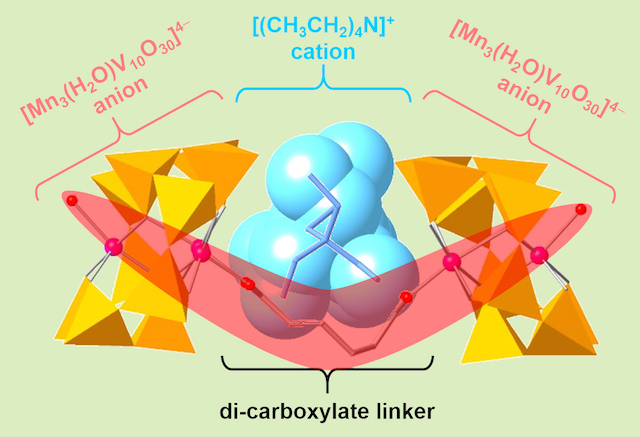
Two anions naturally repel each other. We have challenged to forcefully connect two -4 anions by a short alkyl chain. Instead of an expecting straight alkyl chain by the result of the repulsive interaction, the alkyl chain was bented to bring two anions actually closer by sandwiching a cation, because the packing pattern minimizes the anionic repulsion. The V-shaped double-anion binds an alkyl ammonium cation through noncovalent interaction.
The vanadium-oxide bowl catches a halide ball then changing a shape to secure it, no longer reactive to an outside reagent. Play a catch, do not fumble a ball, like in a baseball game. The inorganic bonds are flexible enough to dynamically change bond angles and lengthes in a totally different isomeric structure––from an open-bowl to a closed-shape just like a move of a mitt in baseball. The process is reversible and you can play it again.
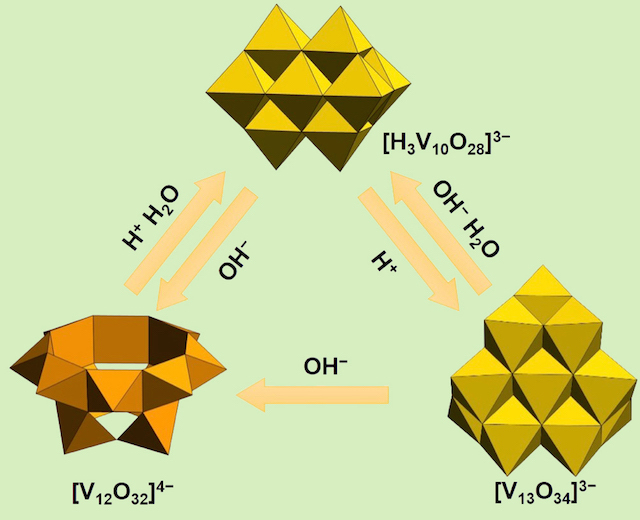
It all appears to be similar in formula. We establish the interconversion reactions among these complexes. The beauty of the ionic complexes in the synthesis is the flexibility of a molecular structure as well as a formulation. You may wonder the stoichiometry does not allow the reaction in the sense of organic chemistry. In inorganic compounds, the bonds are consisted by much weaker ionic bonds and it allows scrumbling of the molecular formura itself. The terminal hydroxido group attacks neigboring molecule, prompting a water molecule kicking out to decrease the number of vanadium atoms or a water addition reaction to produce oxido- or hydroxido-briges to increase the number of vanadium atoms. The end result is a spontaneous scrambling of molecular formula, and only thrmodynamically stable forms survive in the reaction similar phenomena to a supramolecular synthsis.
The review on isopolyoxovanadate species including reduced species is presented in Japanese language.
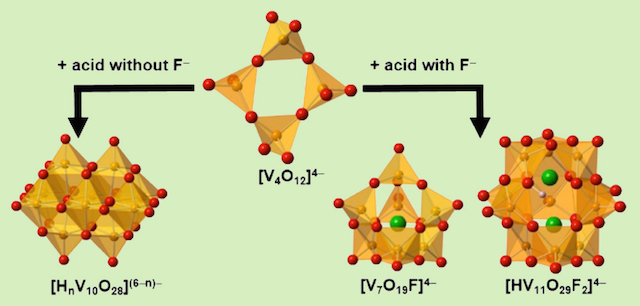
In aqueous polyoxovanadate chemistry, the only polyoxovanadate that is stable in an acidic condition is the decavanadate species, [V10O28]6-. No other species is stable in acidic aqueous solution. We discover that a presence of fluoride ion stimulates the equilibrium in a mixed-aqueous solution and produces a new fluoro-polyoxovanadate species.
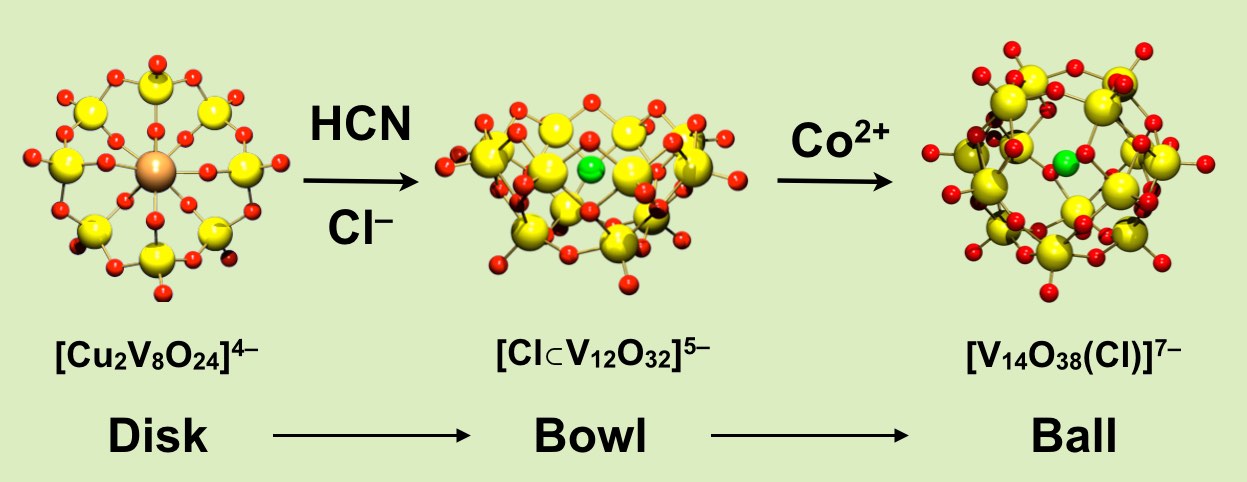
"Umpolung" in chemistry changes a reactivity. The polarity change from a cation-guest complex to an anion-guest complex was utilized to synthesize a ball-molecule stepwise with a guest. Starting from a disk-type inorganic complex of a cationic guest, the elimination reaction of metal cations by cyanide gas under the presence of a specific anion produces an anion-incorporated bowl. It ultimately constructs a ball-type molecule with a guest anion enclosed inside. The rational synthesis of a ball molecule with a particular guest was achieved starting from a bowl, putting a guest in the ball, then it completes the construction of a ball in stepwise manner: the way to allow you to incorporate your favorite guest in the ball.
Catalytic oxidation rates increase in a strong acidic condition and a catalyst has to survive. We investigated a polyoxovanadate species that was produced in such a very strong acidic condition. The isolated descrete hexadecavanadates have a reduced core yet stable under air in nonaqueous solution showing multistep reversible redoxes.
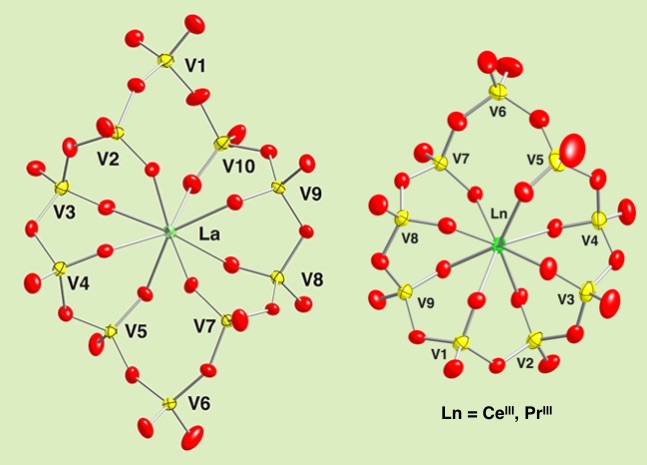
The macrocyclic polyoxovanadate rings incorporate transition matal cations at its center acting as a ligand. When the ionic radius of the metal cation increases up to Lanthanum(III), what happen was the expansion of the vanadium ring size and it was able to adopt a series of big catioins, La(III), Ce(III) and Pr(III). Nine and ten membered-vanadium rings capture those big cations, and the ring shows fluxional behaviour in solution monitored by temperature-dependent 51V NMR.
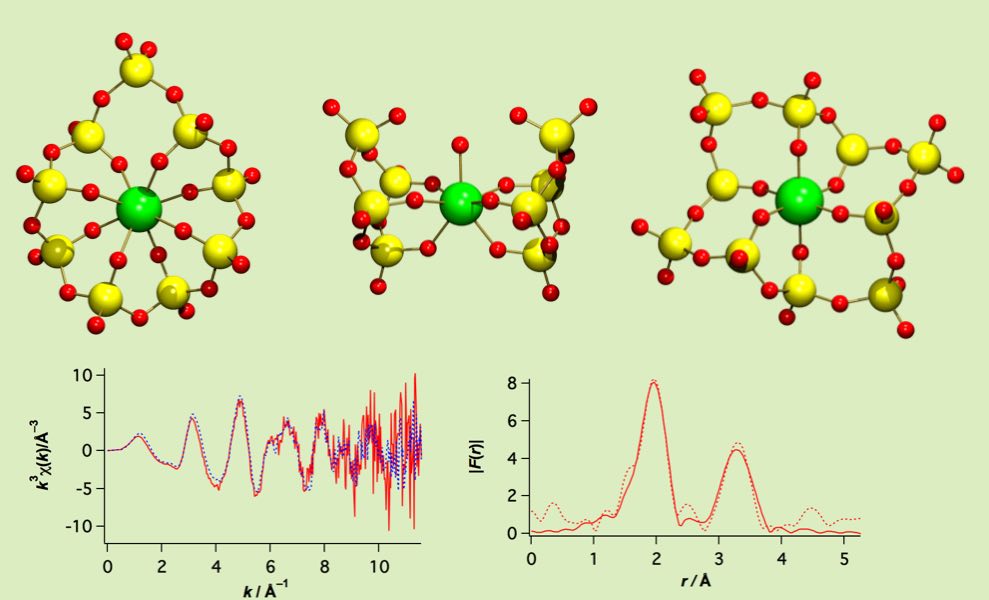
The discrete lanthanide complexes of all-inorganic system have a polyoxovanadate-ring ligand. The VO4 unit on the ring allows to adjust two coordination parameters: i) the various coordination bite-angles by adjusting ring conformation, ii) the variable coordination donor numbers through whether each unit coordinates or not. By alternating the coordination modes, it adopts to fit to the ionic-radious range of all lanthanide cation series. The ionic complexes keep its structure even in the solution that was confirmed through EXAFS study.
The Dawson-type framework may construct from a well available starting material, a rubust hexamolybdate. Hexamolybdates are the most fundamental polyoxometalate with six molybdenum(VI) cations arrnaged in Oh symmetry. It should be a good precursor for a new type of polyoxomolybdates, but was too stable in organic solvent. "How to destroy it to exploit the readily available stubborn structure?" was a problem. The reduction of the hexamolybdate with acetic acid makes the robust unit rearranged and resulting in an isolation of a new lacunary-type Dawson complexes. It has molybdates, MoO42-, as a hetero-atom center, with capping acetate ligands at the lacunary sites, a reduced hexa-lacunary-Dawson-type complex without north- and south-pole. It is air-sensitive and susceptible to hydrolysis, but it is stable in anaerobic condition.
The review on iso- and heteropolyoxovanadates including lacunary polyoxovanadates.
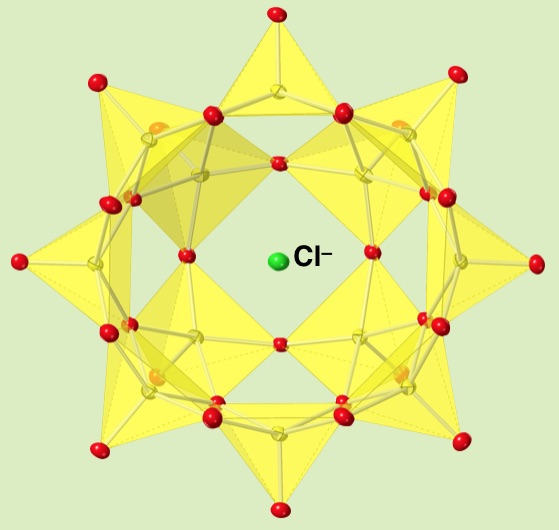
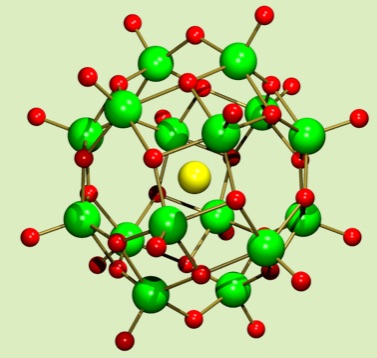
The bowl-type molecule, dodecavanadate, is an inorganic anionic host molecule. The presence of solvent prevented the guest capturing, but we build an inorganic host-guest complex from a precursor by ship-in-bottle type synthesis. Inserting a chloride anion inside a dodecavanadate bowl builds the anion trapped inside the bowl-anion.
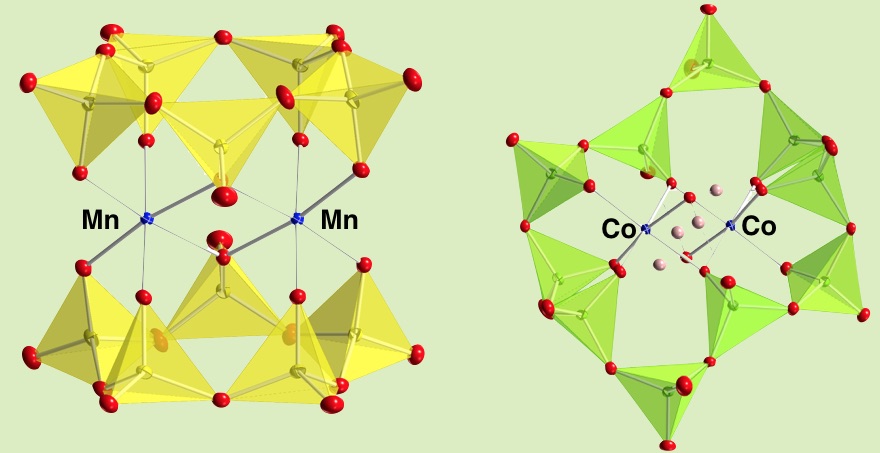
The vanadate rings are a good inorganic ligand and it forms dinuclear complexes of Mn(II) or Co(II). The pentameric vanadate ring was first isolated in this complex.
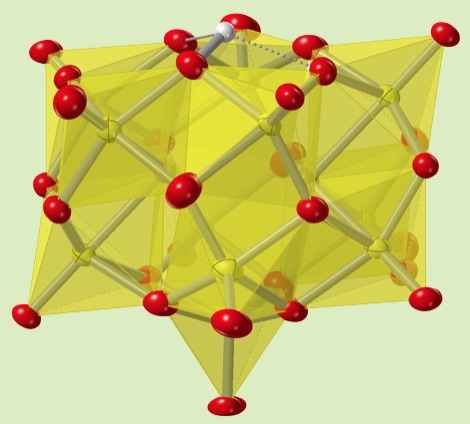
The oxidation reaction of the reduced-decavanadates produces various lacunary-type polyoxovanadates with a guest anion in its spherical structure. The lacunary position was protonated to hold the spherical structure.
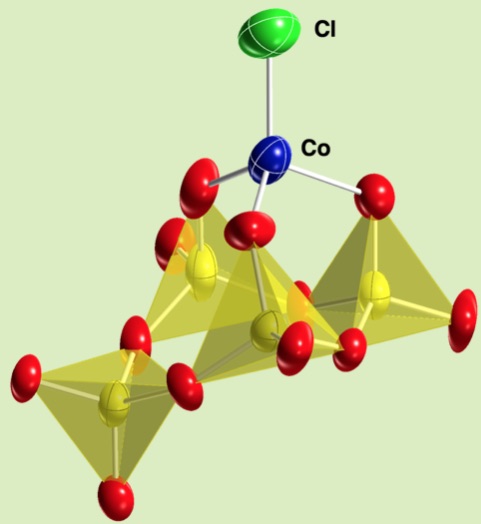
Cyclic Tetravanadates are a major species in nonaqueosu soltion and the one of the most important species in polyoxovanadate chemistry. It can be tridentate ligand against tetrahedral metal cationic unit.
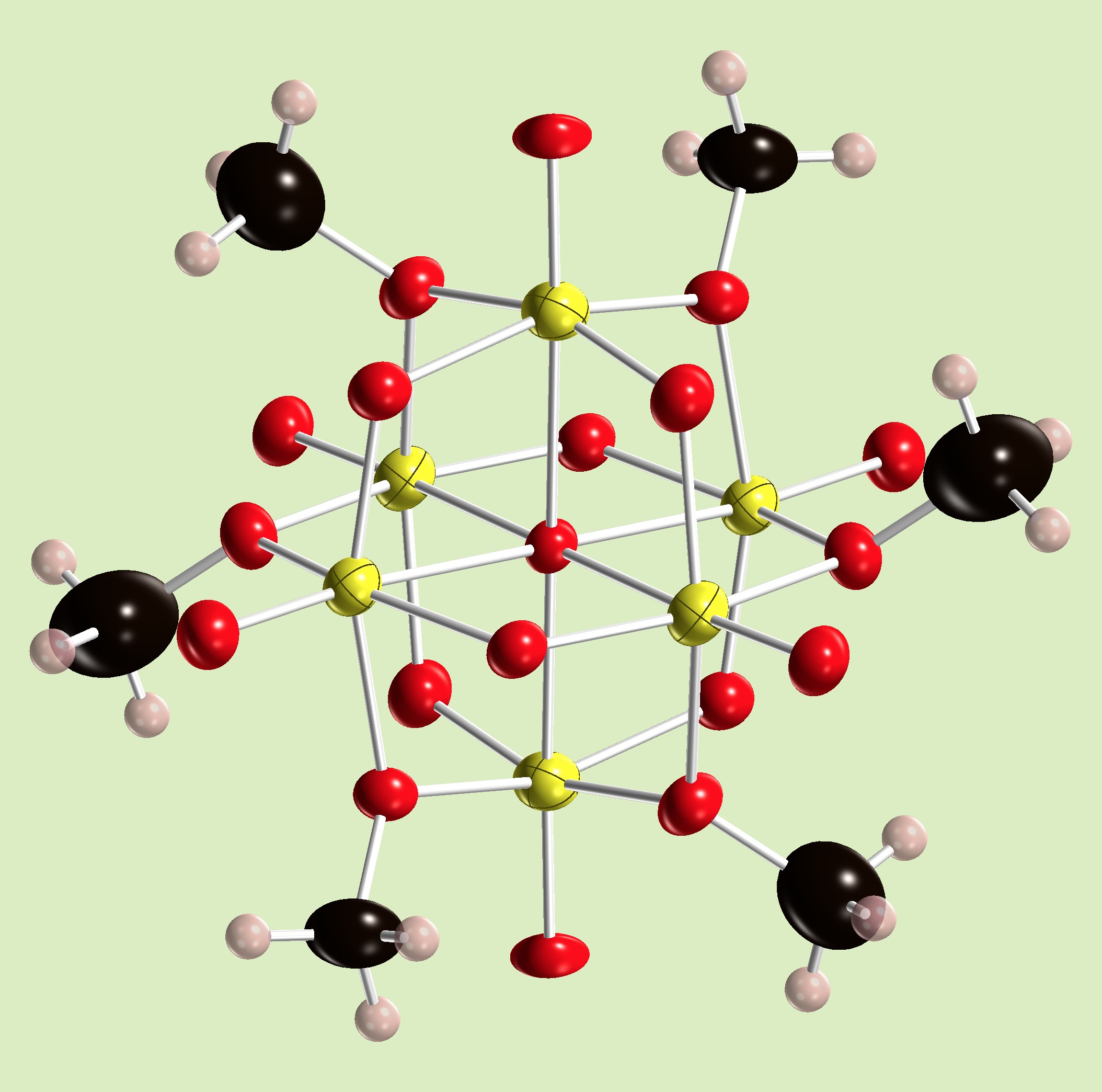
A V(V) hexamer, the Lindqvist-type hexametalates is a representative polyoxometalate framework. It has labile methoxo-groups on the core. Those methoxo groups are moving rapidly on the surface, however in the solid state, it prefers a pseudo-hexagonal arrangement on the hexametalate core. It produces by disolving the precursor in methanol.
